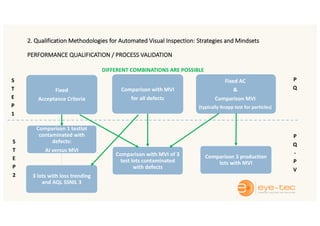
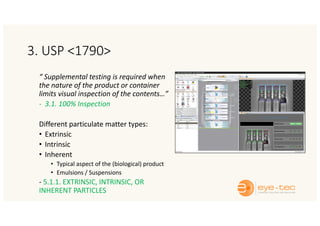
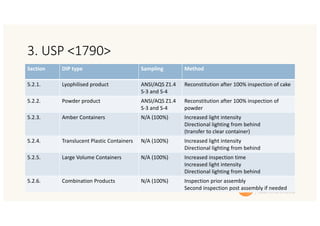
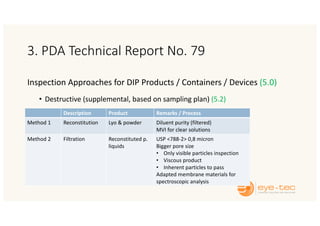
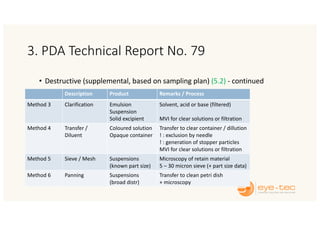
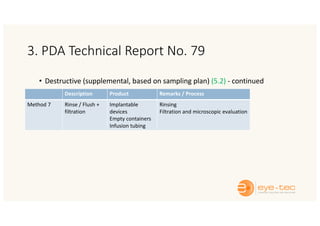
The document outlines strategies and methodologies for automated visual inspection in quality control, focusing on defect categories (critical, major, minor) and their implications for patient safety. It details qualification processes for automated systems, including risk assessments, performance qualification, and comparison with manual inspection methods. Additionally, it addresses challenges in inspecting difficult products, proposing enhanced testing and control strategies to ensure product integrity.