The document summarizes findings from a large survey and qualitative research exploring public knowledge and attitudes towards medicines research and development (R&D) in six European countries. Key findings include:
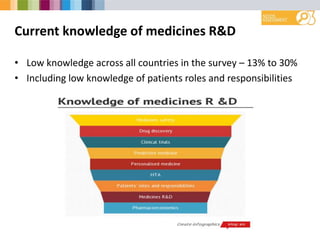
- Low overall knowledge about medicines R&D across countries, ranging from 13-30% knowledgeable.
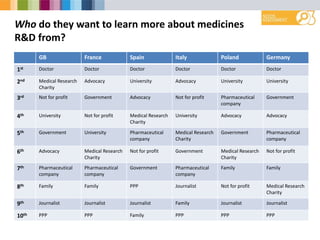
- Varied levels of trust in different R&D stakeholders between countries.
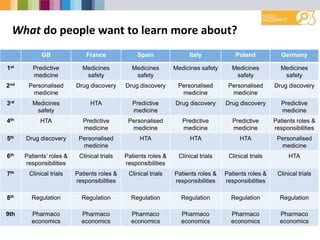
- Greatest interest in learning more about medicines safety, personalized medicine, and drug discovery.
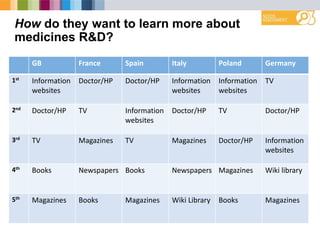
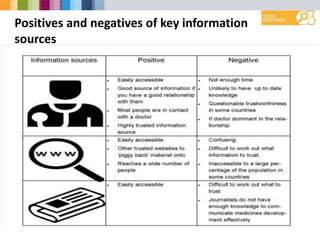
- Preferred sources of information are doctors, websites, and television.
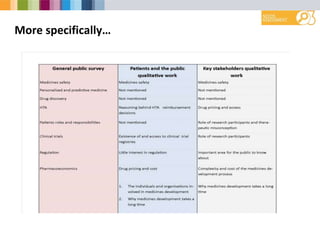
- Suggested focus areas for public engagement include the R&D process, clinical trials, regulations, and patient roles.