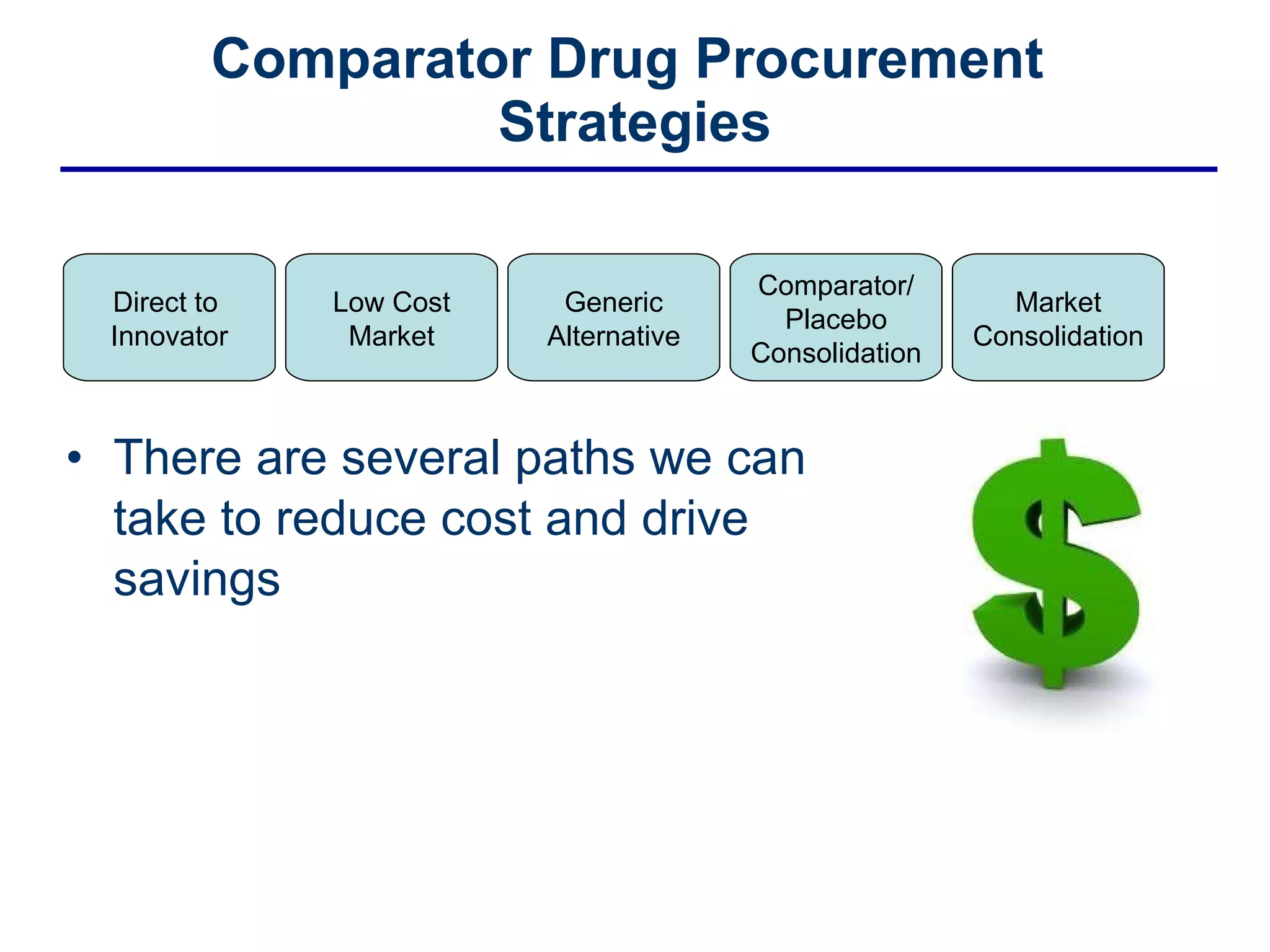
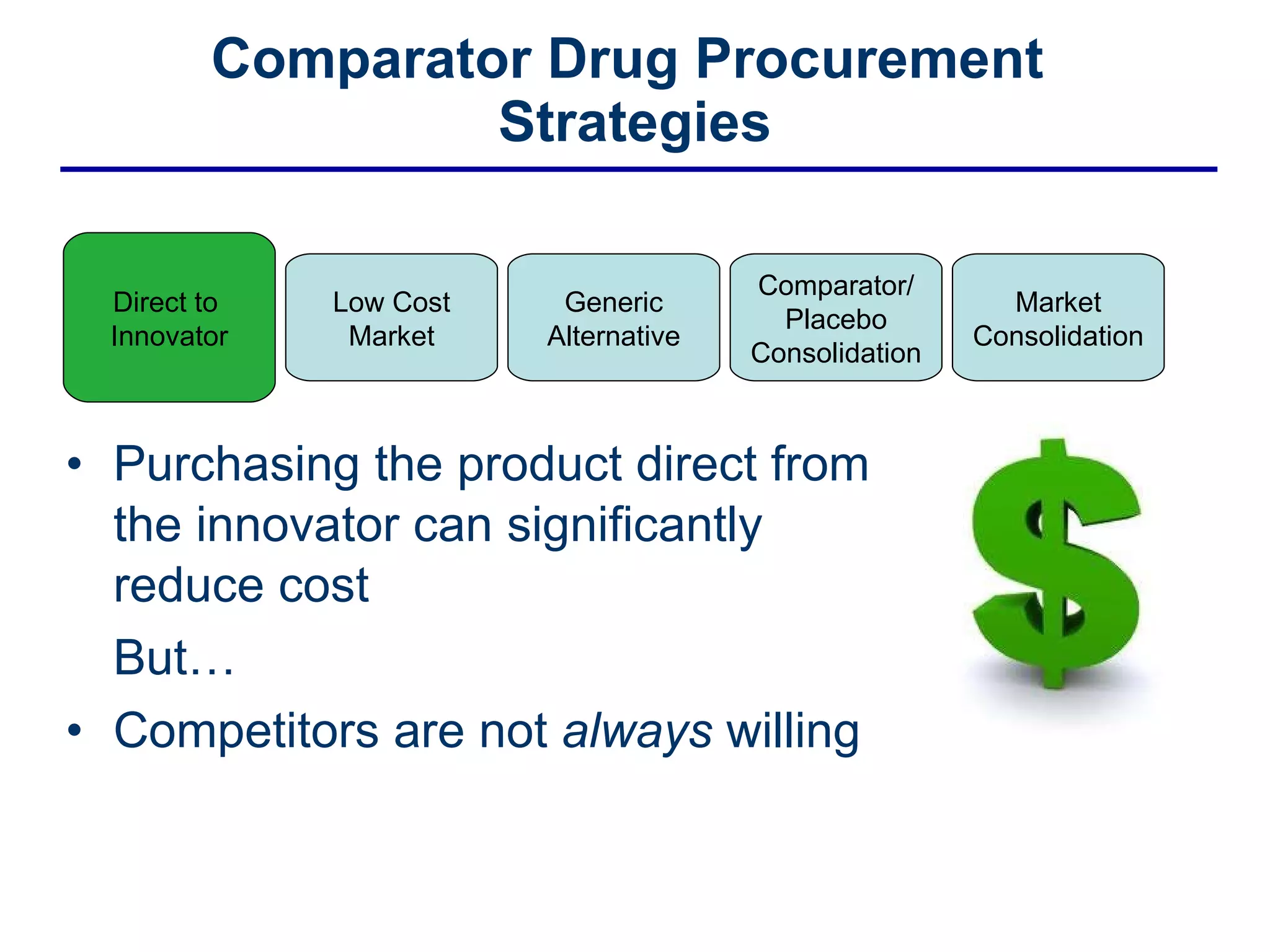
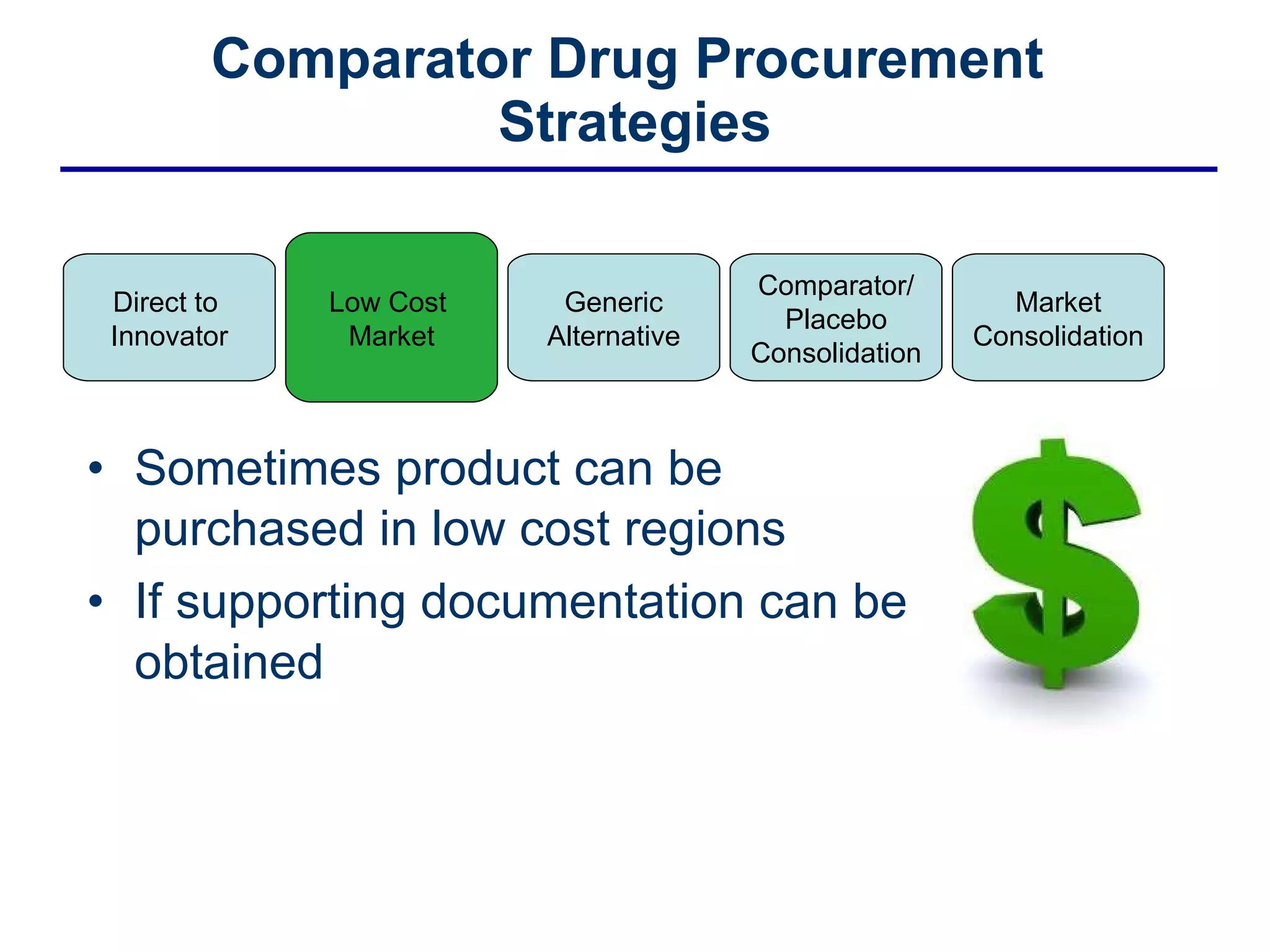
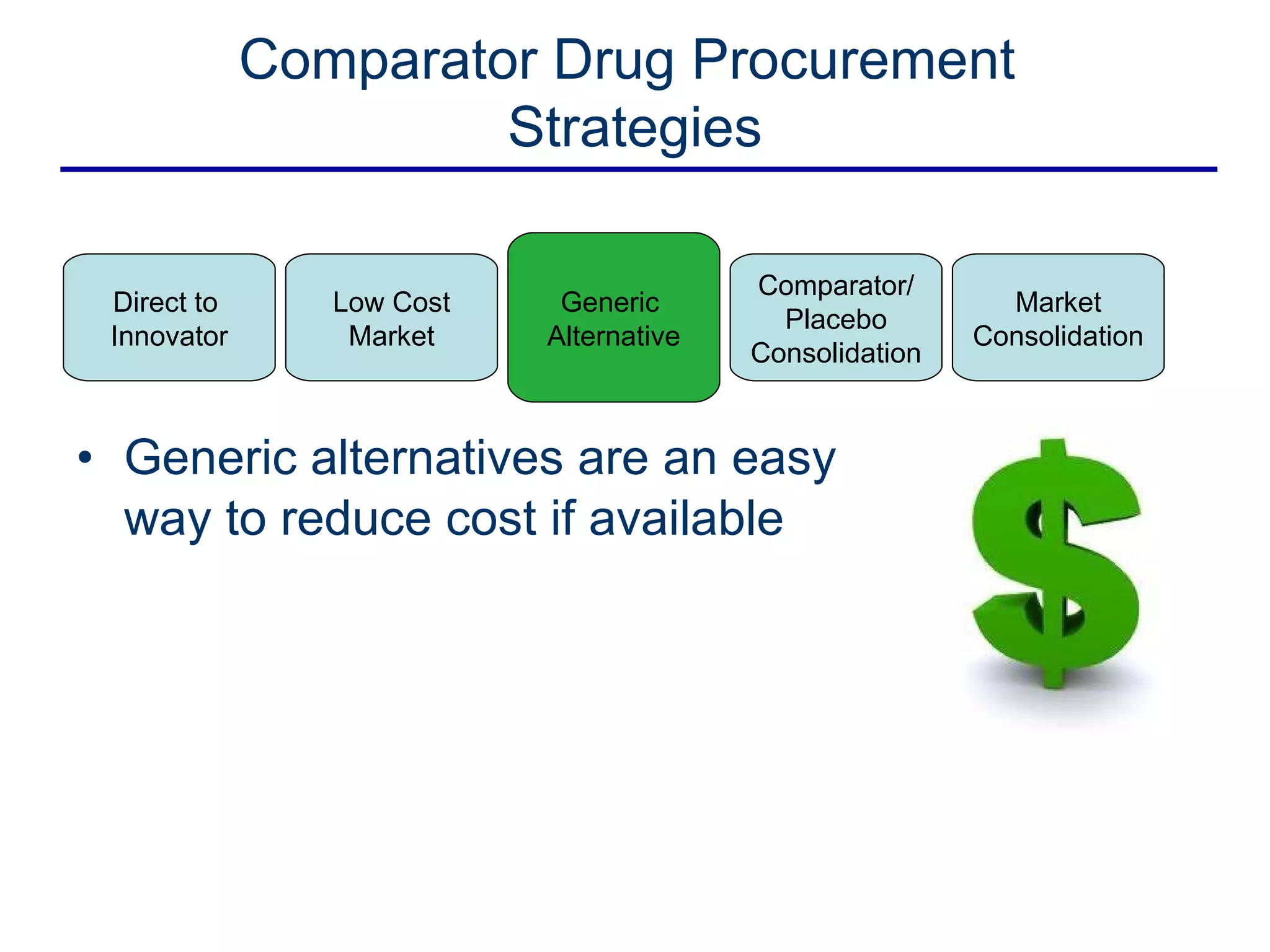
This document discusses the challenges of sourcing comparator drugs for clinical trials and strategies for reducing costs. It outlines regulatory requirements, industry changes, and supply chain complexities that make comparator drug procurement difficult. The document then presents several strategies for reducing costs, such as sourcing directly from innovators, procuring from low-cost markets, using generics, consolidating comparators and placebos, and coordinated supplier relationship management.