Embed presentation
Download as PDF, PPTX



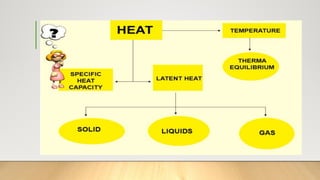
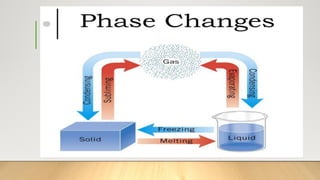







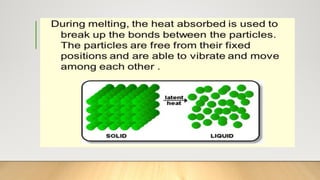


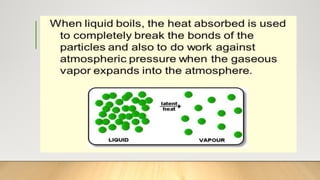
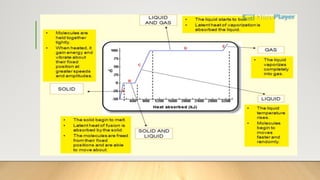
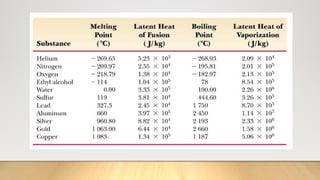
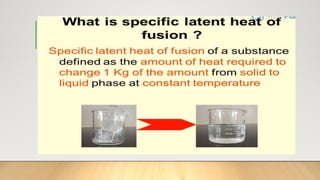








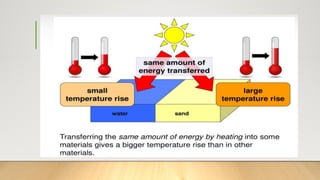

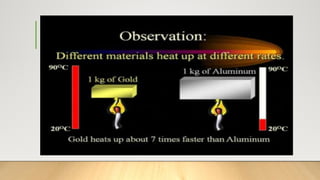
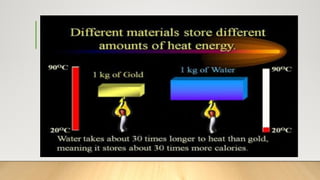


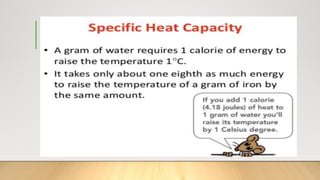

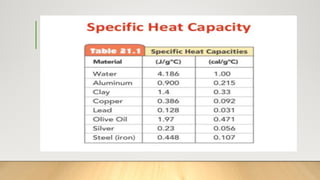





The document discusses latent heat and specific heat capacity, specifically calculating the energy required to melt 450g of ice and to raise the temperature of 0.15kg of water from 20°C to 55°C. It uses the formula q=mcΔt to determine that 21,976.5 joules (or 22 kJ) are needed to heat the water. The calculations illustrate the concepts of heat transfer in materials.











































