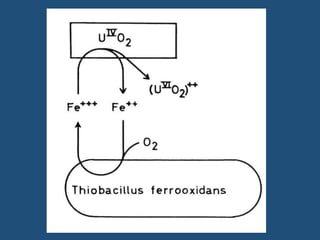
Microorganisms play a key role in transforming micronutrients in soil. Bacteria oxidize iron from the ferrous to the ferric state, precipitating it out as iron hydroxide. They also solubilize zinc, making it more available to plants. Various bacteria and fungi transform manganese and copper in the soil through oxidation and precipitation reactions. These microbial transformations influence the availability and forms of essential micronutrients for plant uptake.