Embed presentation
Download as PDF, PPTX
![Department
of
Chemistry
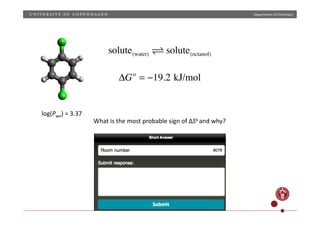
PbI2(s) Pb(aq)
2+
+ 2I(aq)
−
K = [Pb2+
][I−
]2
ΔGo
= 46.1 kJ/mol ⇒ [Pb2+
] = 1.14 ×10−3
M
measured: [Pb2+
] = 1.37 ×10−3
M
K = e−ΔGo
/RT
Explain
the
difference](https://image.slidesharecdn.com/questionssa-140618073539-phpapp01/85/Short-answer-questions-on-thermodynamics-1-320.jpg)
![Department
of
Chemistry
CH3COOH CH3COO−
+ H+
pKa = −log Ka( )
Ka =
[CH3COO−
][H+
]
[CH3COOH]
the
pKa
in
water
is
4.76.
Is
the
pKa
higher
or
lower
DMSO
and
why?
S
ε
=
47](https://image.slidesharecdn.com/questionssa-140618073539-phpapp01/85/Short-answer-questions-on-thermodynamics-3-320.jpg)
The document discusses various chemical equations and thermodynamic properties related to solute interactions, ligand binding, and the significance of free energy changes (ΔG°) and entropy changes (ΔS°). It includes specific reactions involving lead ions (Pb2+) and acetate ions (CH3COO−), detailing their equilibrium constants and pKa values in different solvents. Additionally, it mentions the positive value of ΔS° in the context of enzyme interactions, indicating an increase in disorder during these processes.
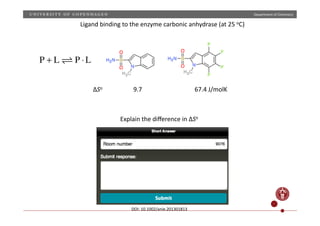
![Department
of
Chemistry
PbI2(s) Pb(aq)
2+
+ 2I(aq)
−
K = [Pb2+
][I−
]2
ΔGo
= 46.1 kJ/mol ⇒ [Pb2+
] = 1.14 ×10−3
M
measured: [Pb2+
] = 1.37 ×10−3
M
K = e−ΔGo
/RT
Explain
the
difference](https://image.slidesharecdn.com/questionssa-140618073539-phpapp01/85/Short-answer-questions-on-thermodynamics-1-320.jpg)

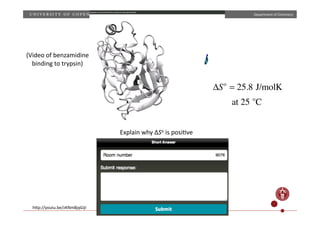
![Department
of
Chemistry
CH3COOH CH3COO−
+ H+
pKa = −log Ka( )
Ka =
[CH3COO−
][H+
]
[CH3COOH]
the
pKa
in
water
is
4.76.
Is
the
pKa
higher
or
lower
DMSO
and
why?
S
ε
=
47](https://image.slidesharecdn.com/questionssa-140618073539-phpapp01/85/Short-answer-questions-on-thermodynamics-3-320.jpg)

