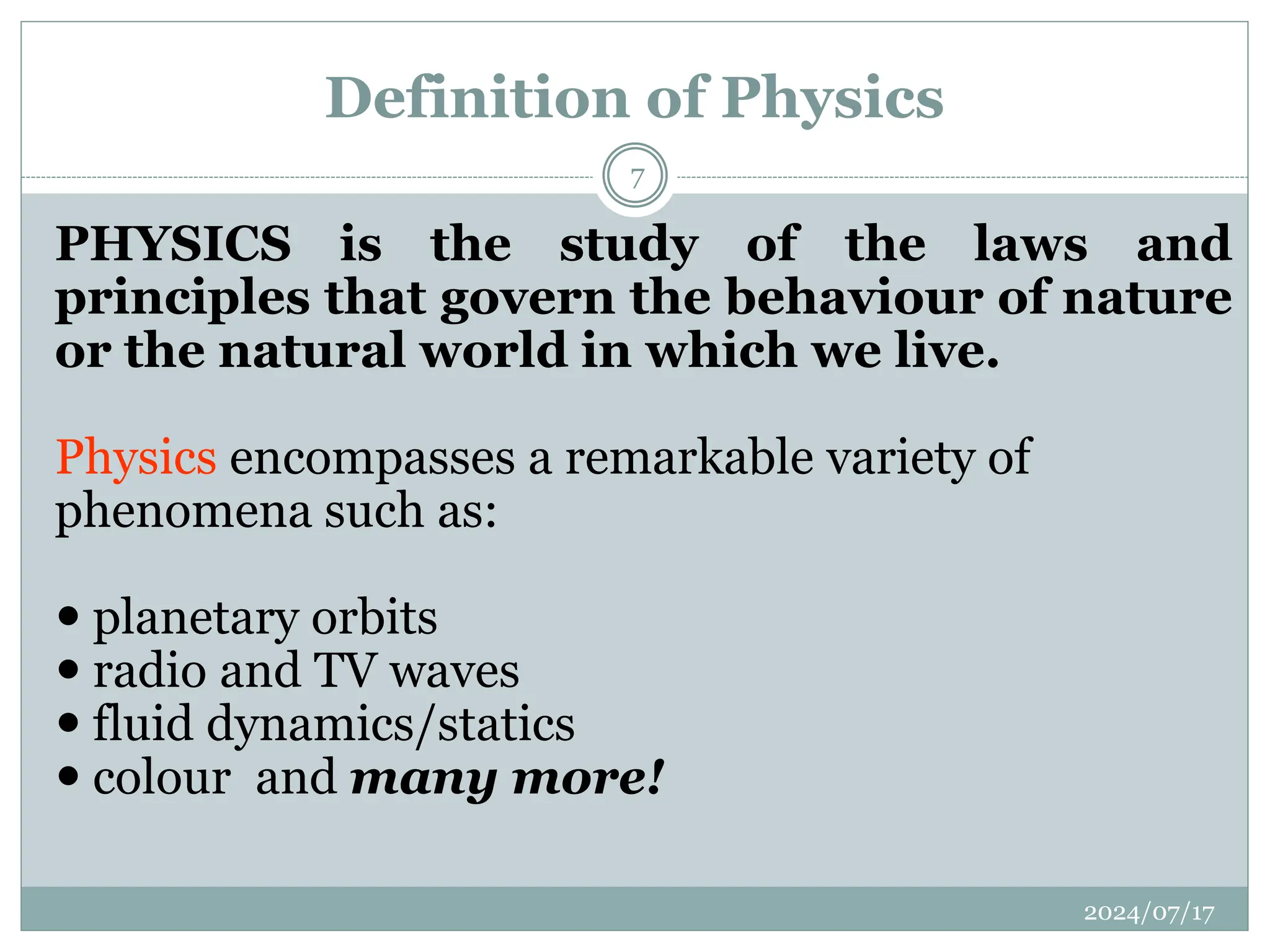
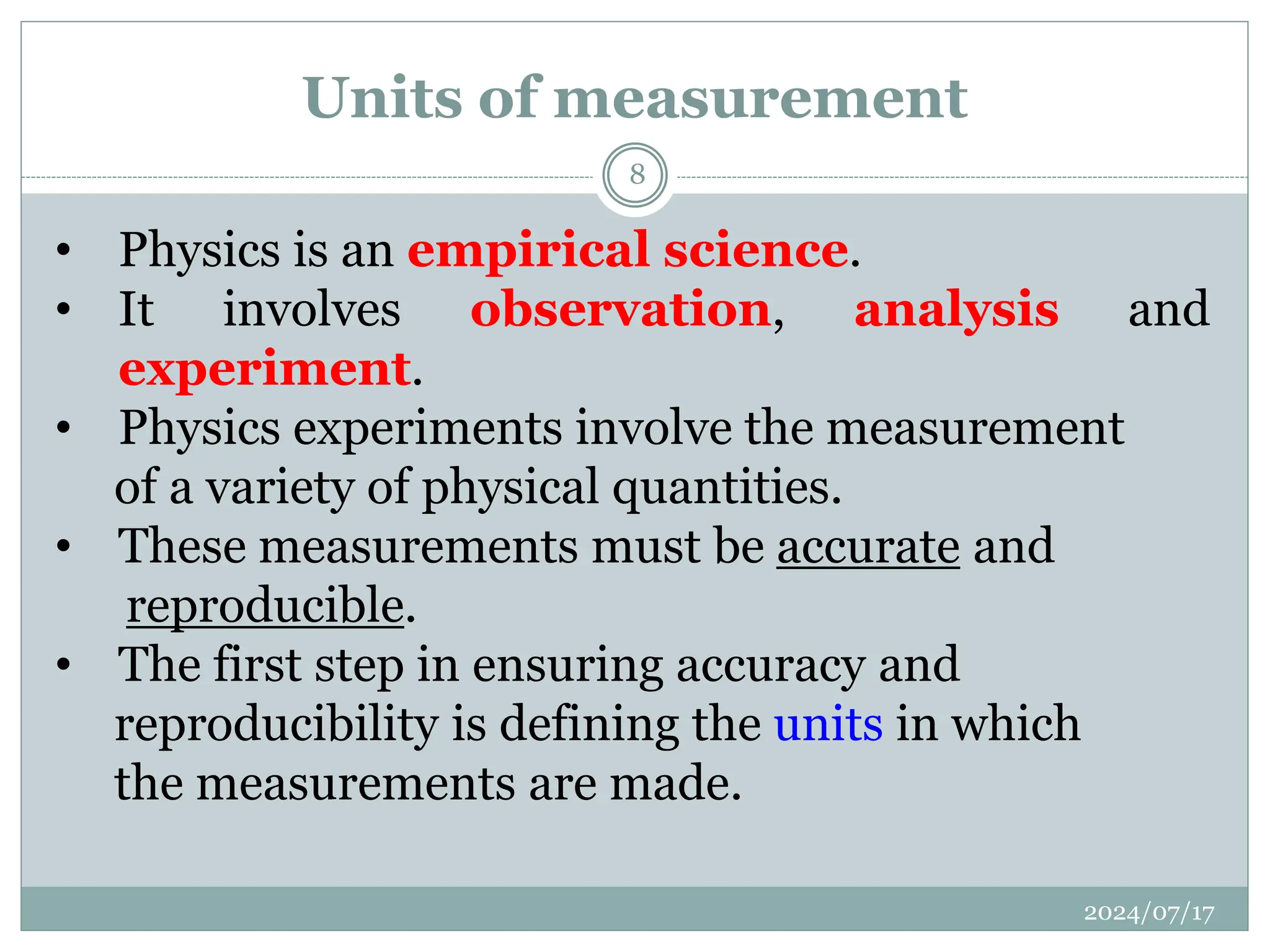
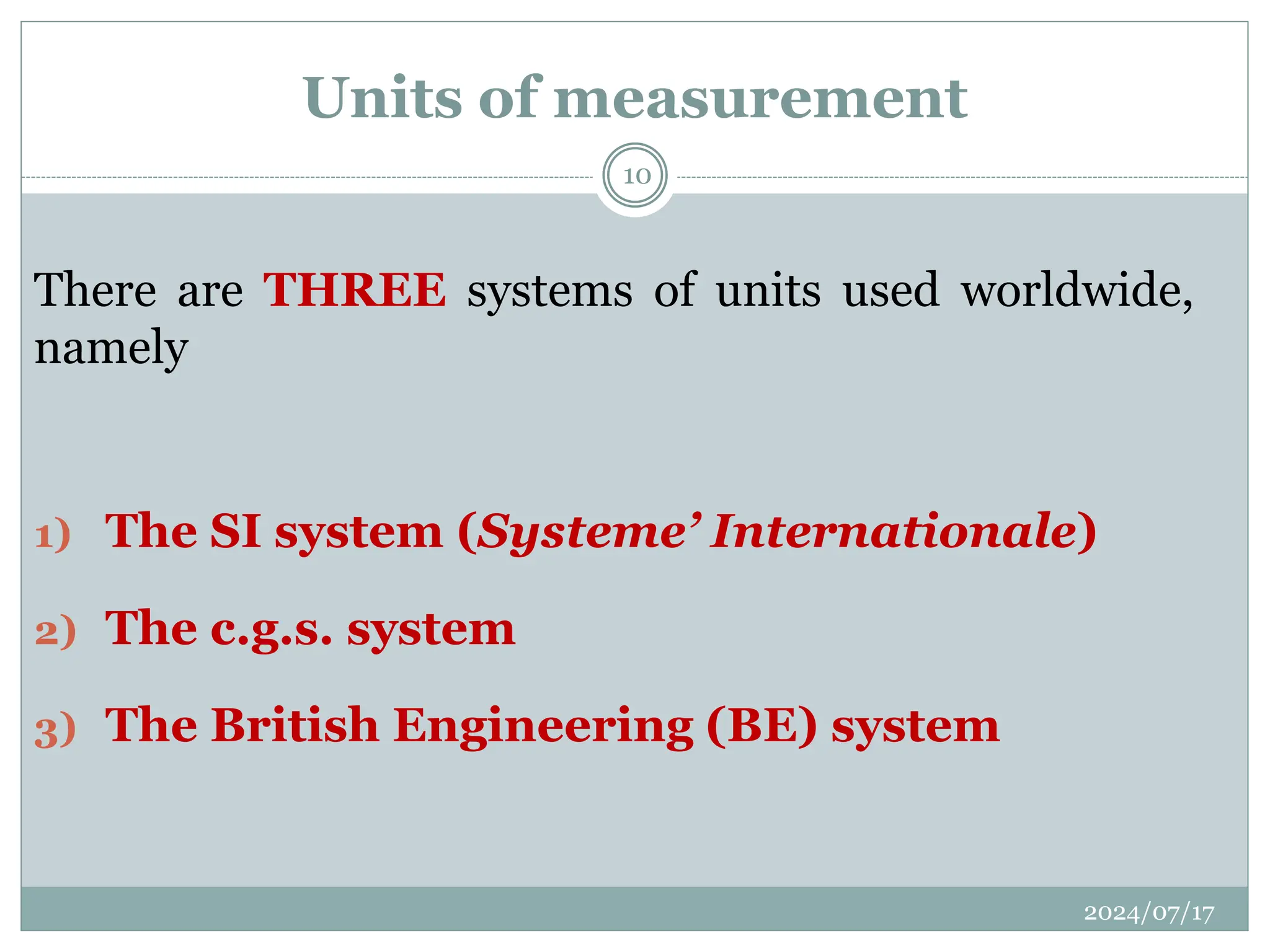
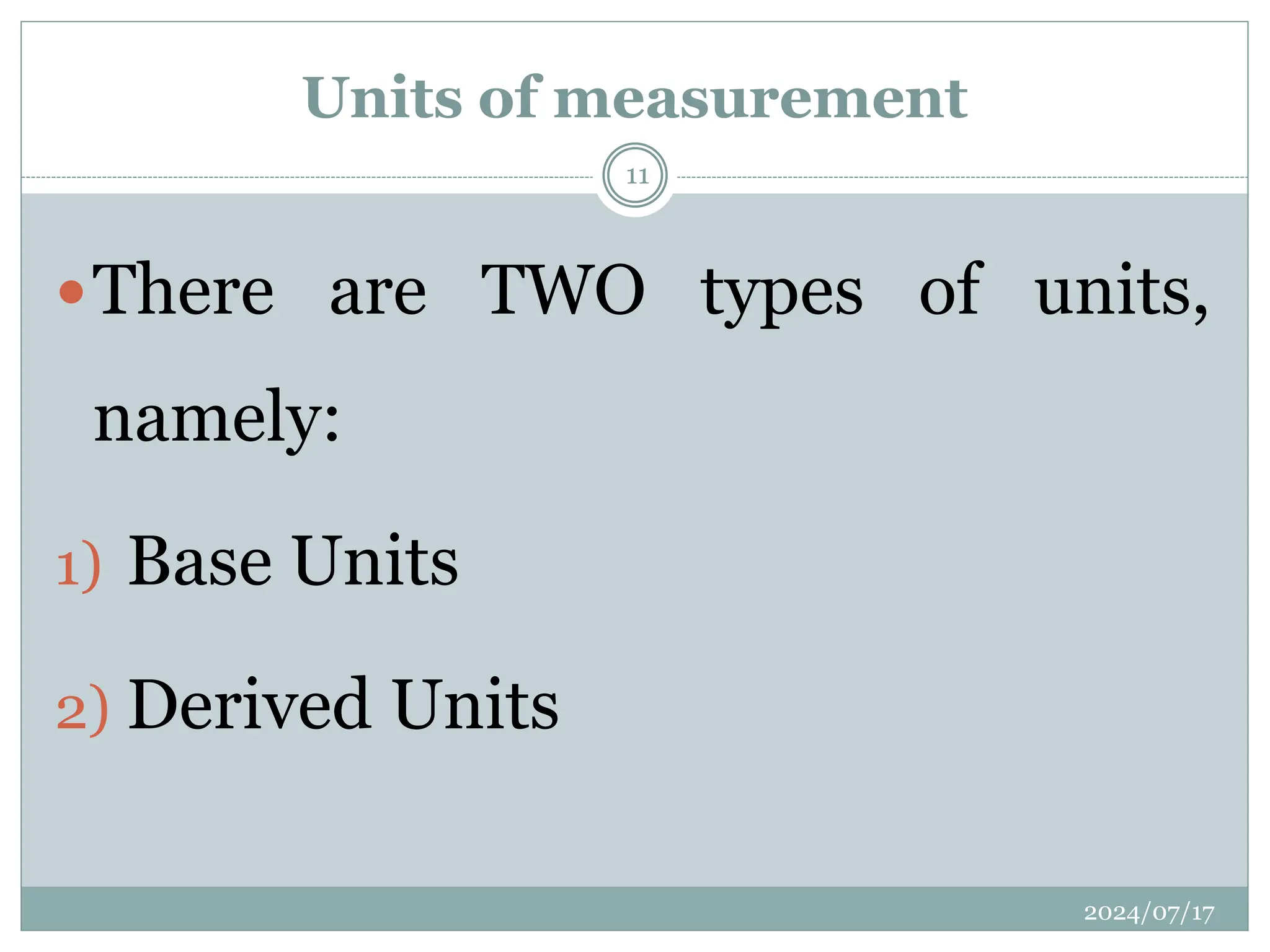
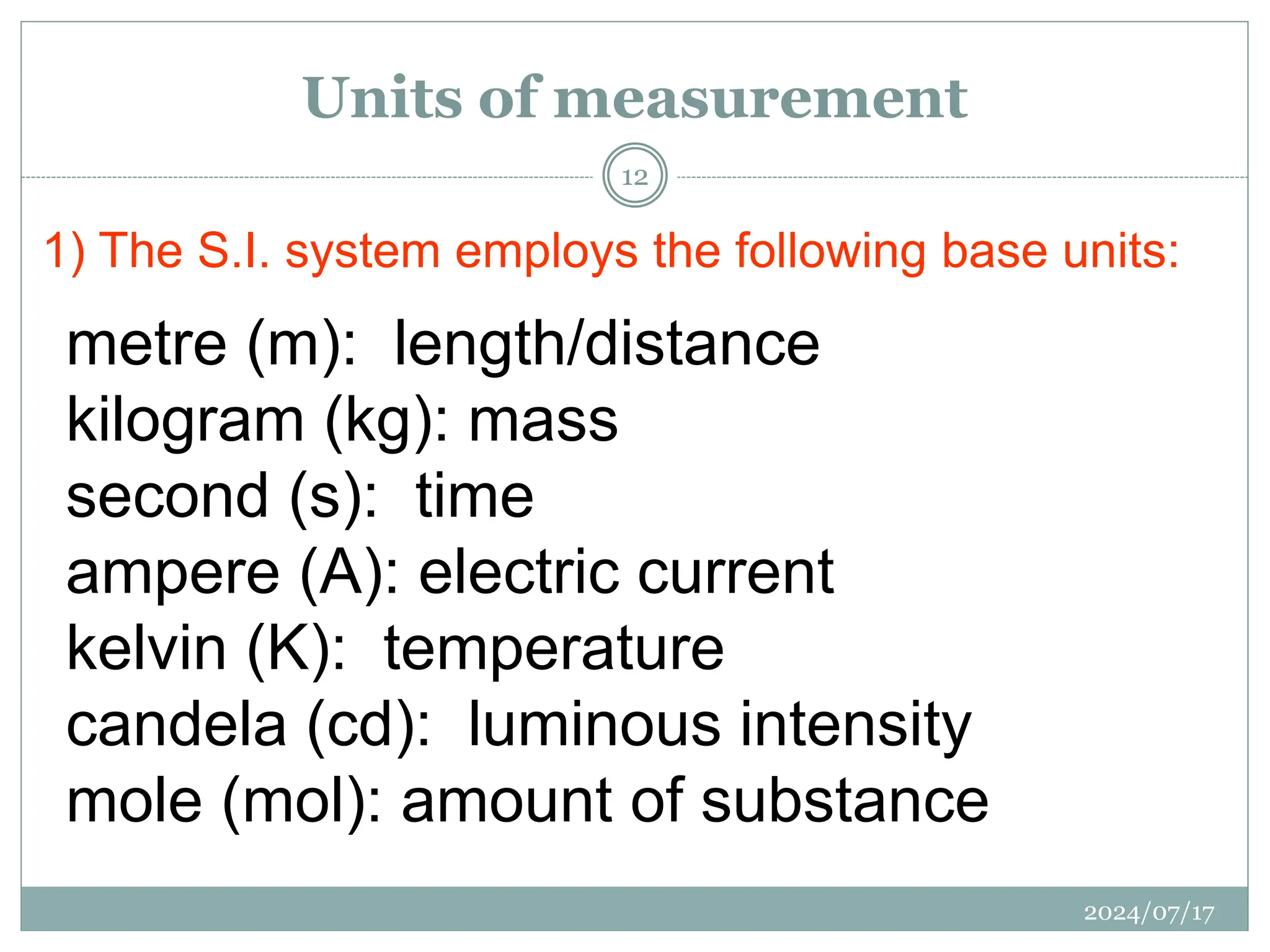
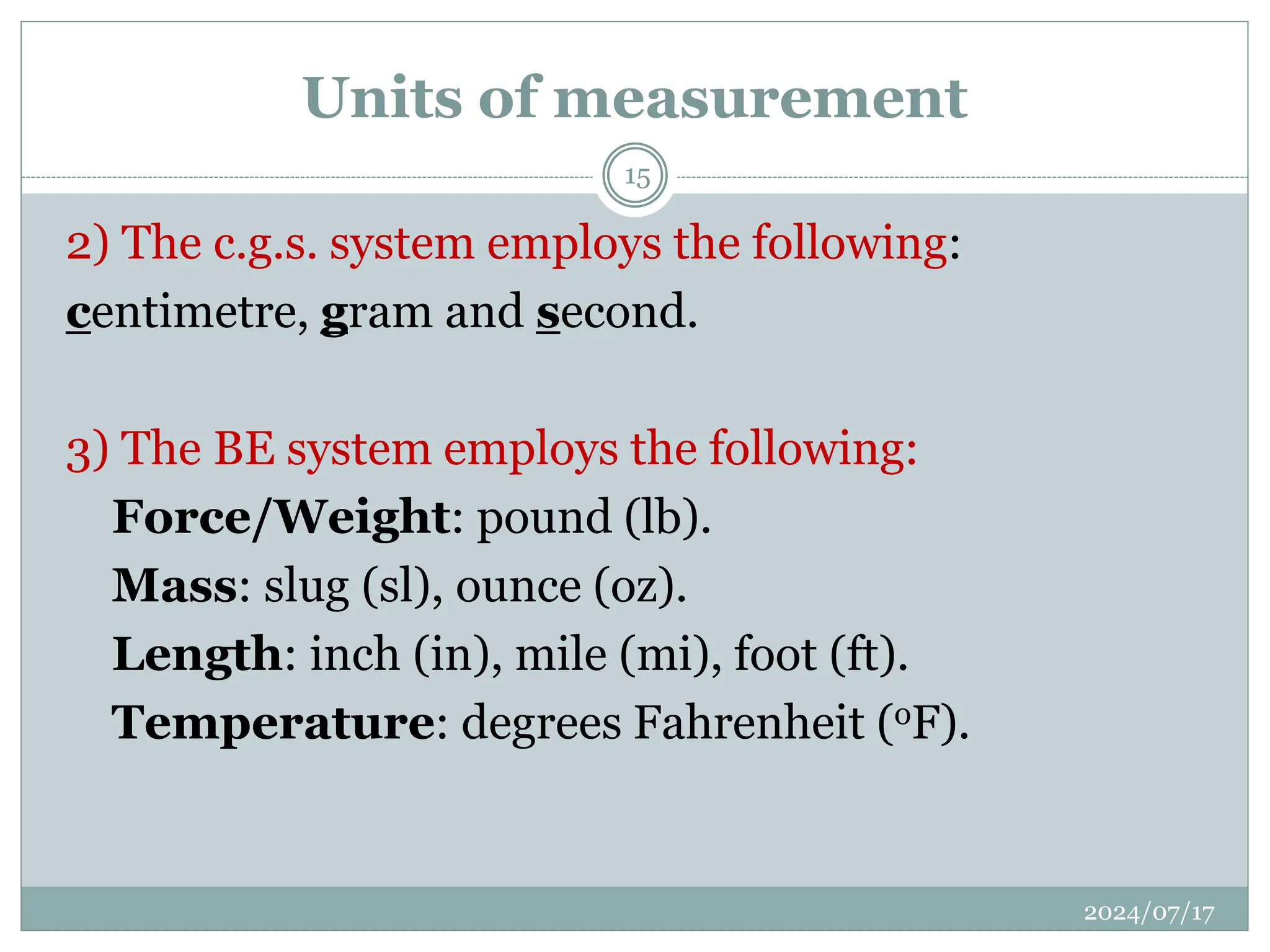
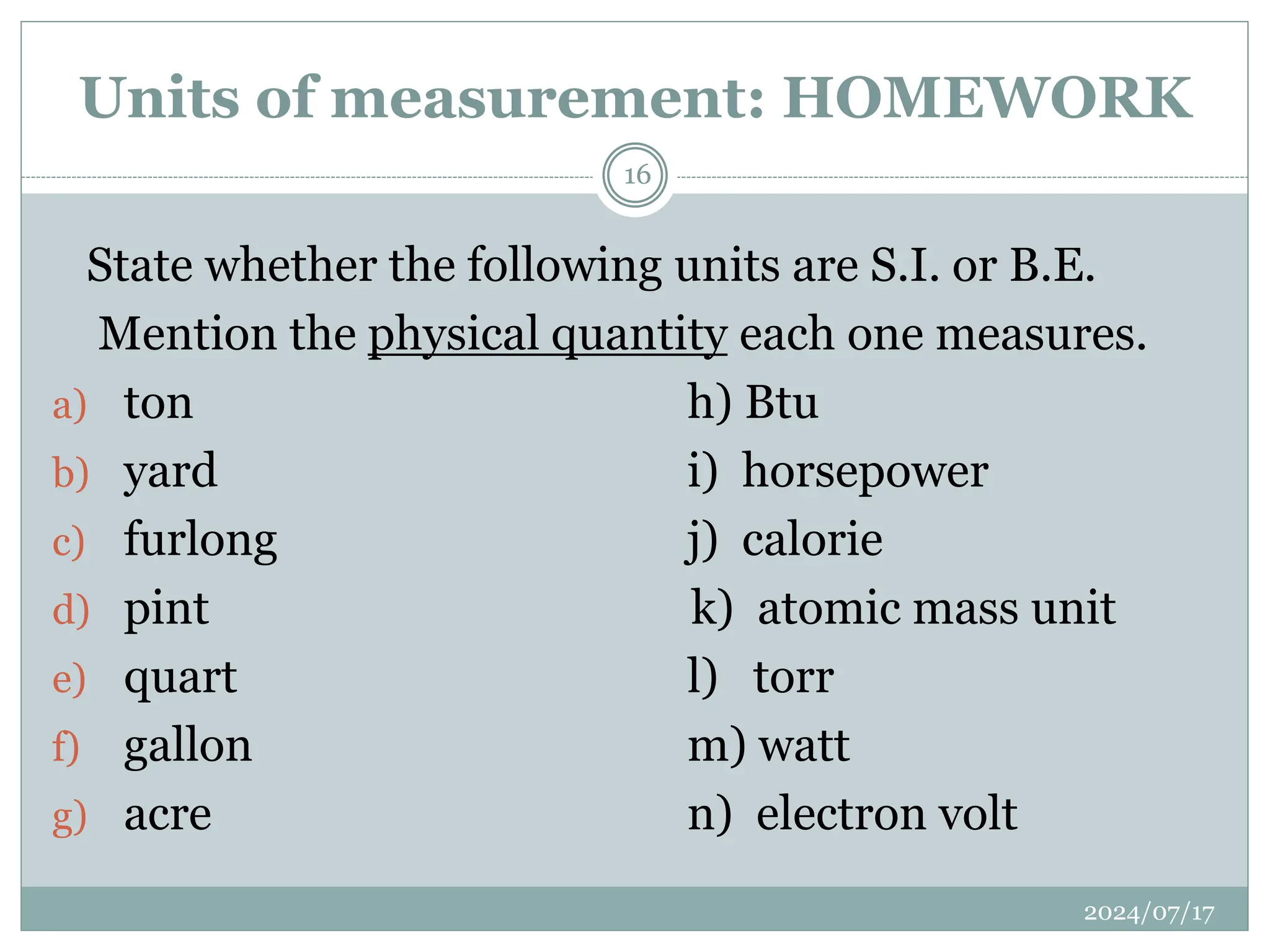
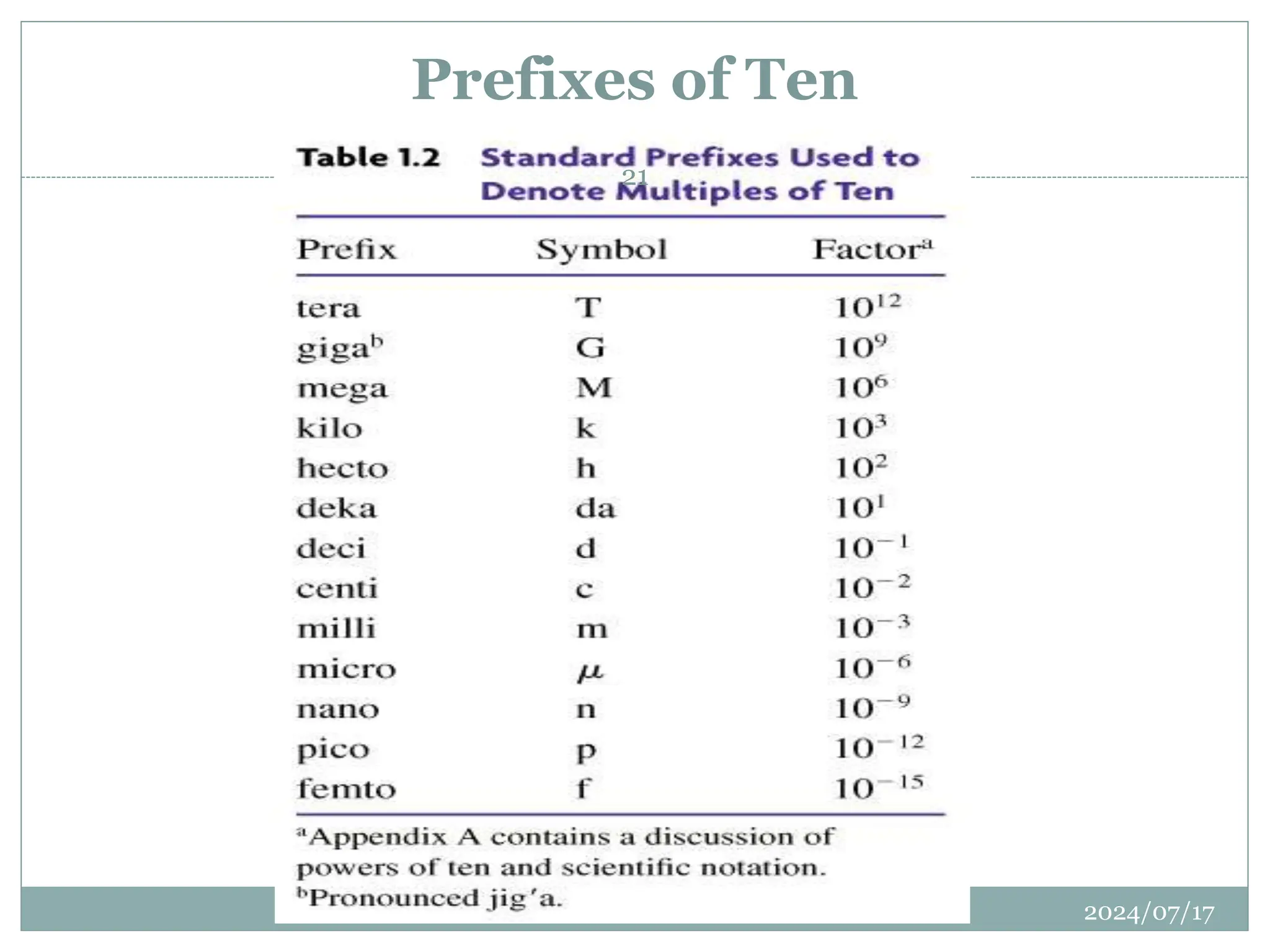
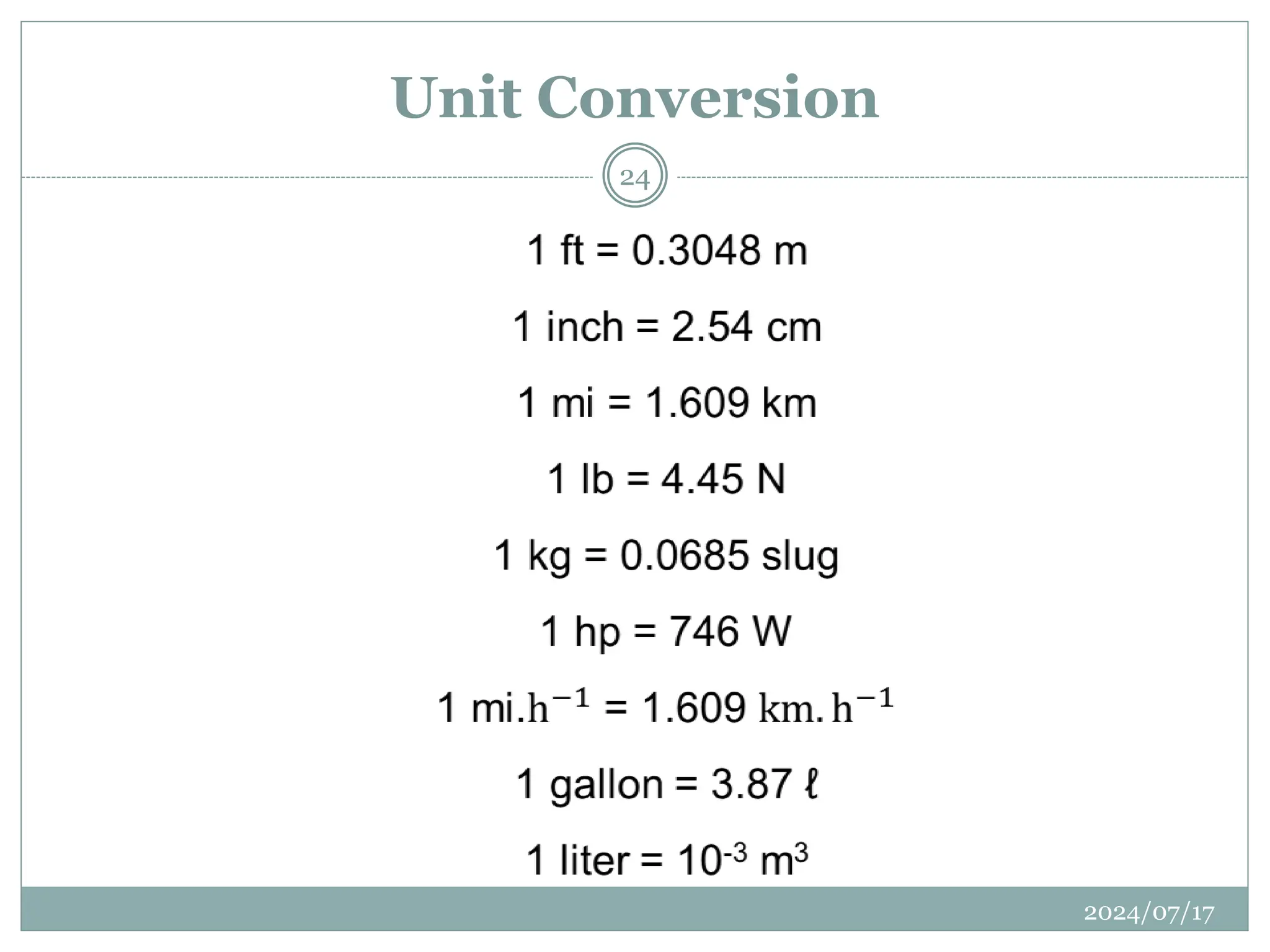
The document outlines the fundamentals of engineering physics, focusing on key topics such as units of measurement, scientific notation, prefixes of ten, trigonometry, logarithms, unit conversion, dimensional analysis, and significant figures. It also provides definitions, examples, and exercises to illustrate these concepts, emphasizing the importance of accuracy and precision in scientific measurements. The tallest building in the world, the Burj Khalifa, is used as a case study to demonstrate unit conversion and measurement in physics.















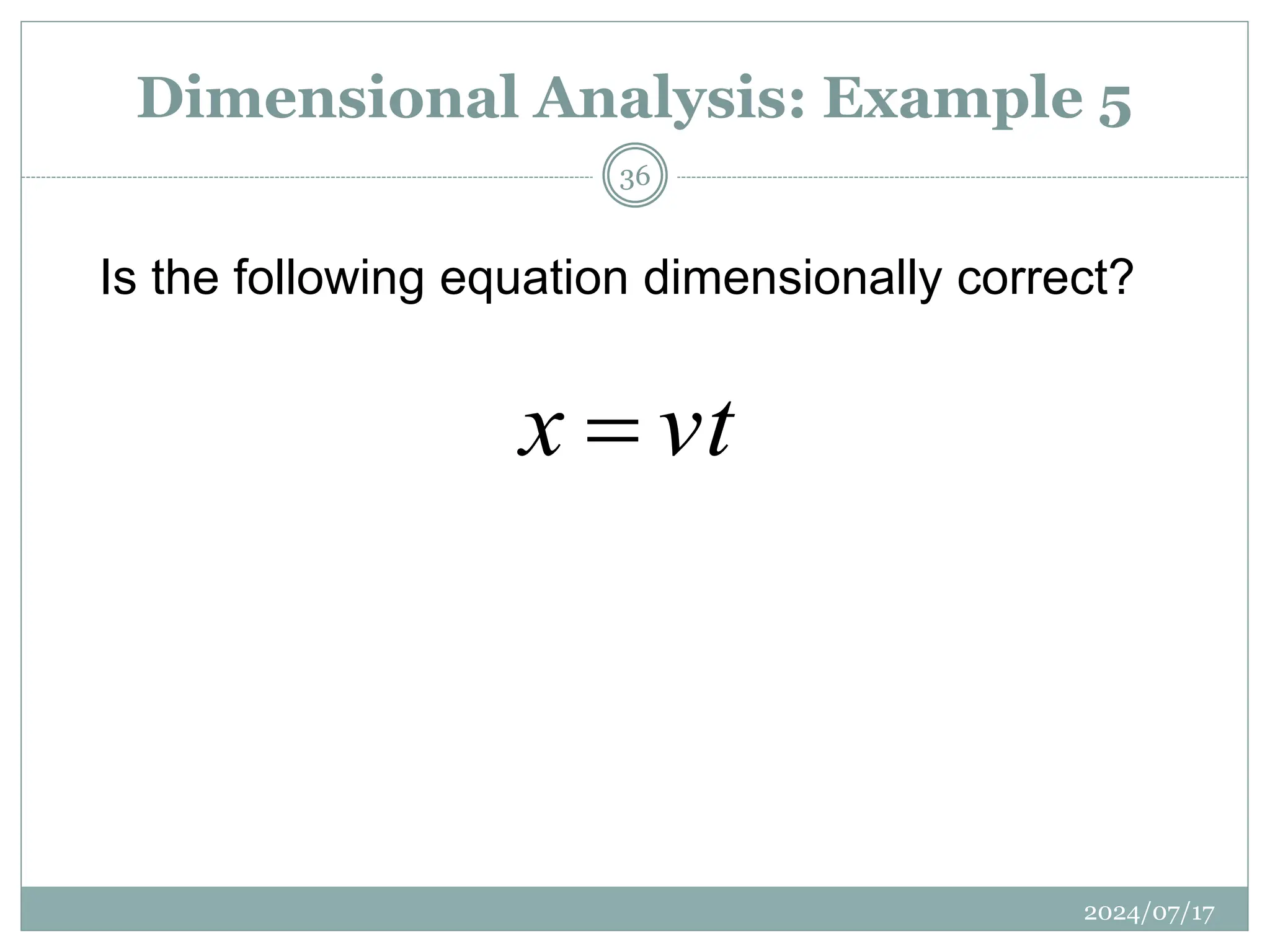
![x= displacement [m],
v = velocity [m.s−1]
t= time [s]
NOTE: The constant
1
2
, must not be considered in
dimensional analysis.
2
2
1
vt
x
Is the following equation dimensionally correct?
Dimensional Analysis: Example 4
2024/07/17
35](https://image.slidesharecdn.com/section1mathematicalbasicsclass1-240717172805-82ba7fa0/75/Section-1_Mathematical-Basics_Class-1-pptx-35-2048.jpg)





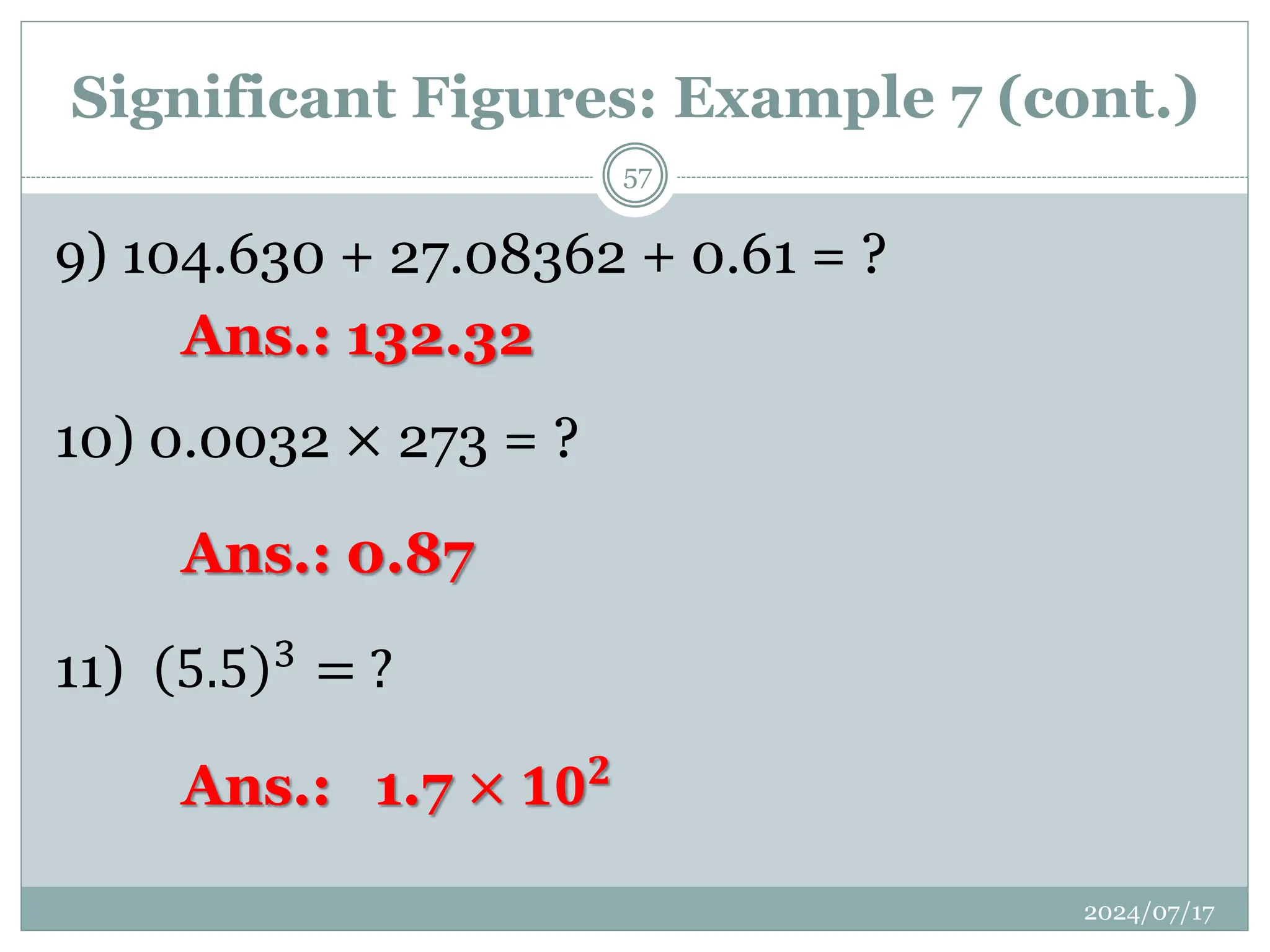
![12) 3.00 × 105 − 1.5 × 102 = ?
13) [(10.3) + (0.01345)] ÷ [(10.3) × 0.01345)] = ?
Significant Figures: HOMEWORK
2024/07/17
58](https://image.slidesharecdn.com/section1mathematicalbasicsclass1-240717172805-82ba7fa0/75/Section-1_Mathematical-Basics_Class-1-pptx-58-2048.jpg)