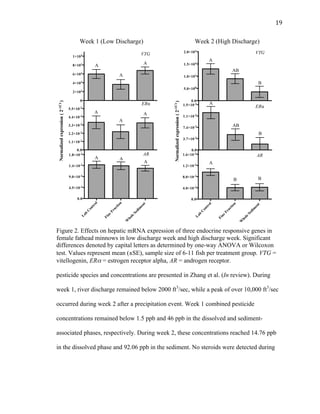
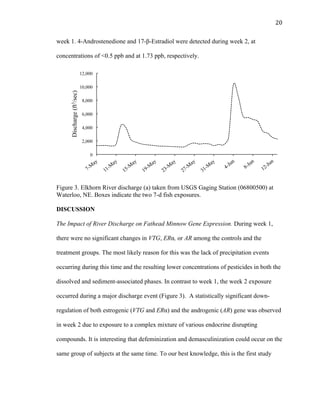
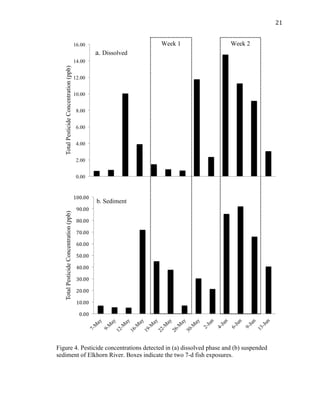
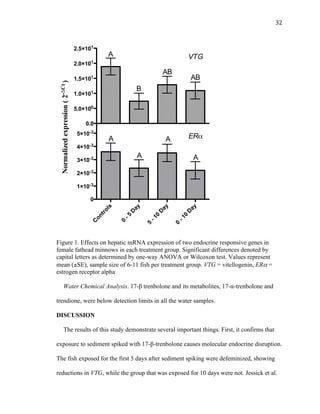
This thesis examines the impact of sediments and endocrine disrupting compounds on fathead minnows through two studies. The first is a field study that exposed fish in mesocosms to water from the Elkhorn River, Nebraska with varying sediment loads during periods of low and high river discharge. Gene expression analysis found reductions in estrogen-responsive genes in fish exposed to full sediment loads only during high discharge. The second study exposed fish to sediment spiked with the synthetic androgen 17-β-trenbolone for 5, 10, or 5-10 days. Fish exposed for the first 5 days experienced molecular defeminization, while longer exposures did not, indicating effects may be reversible at low levels. Overall,