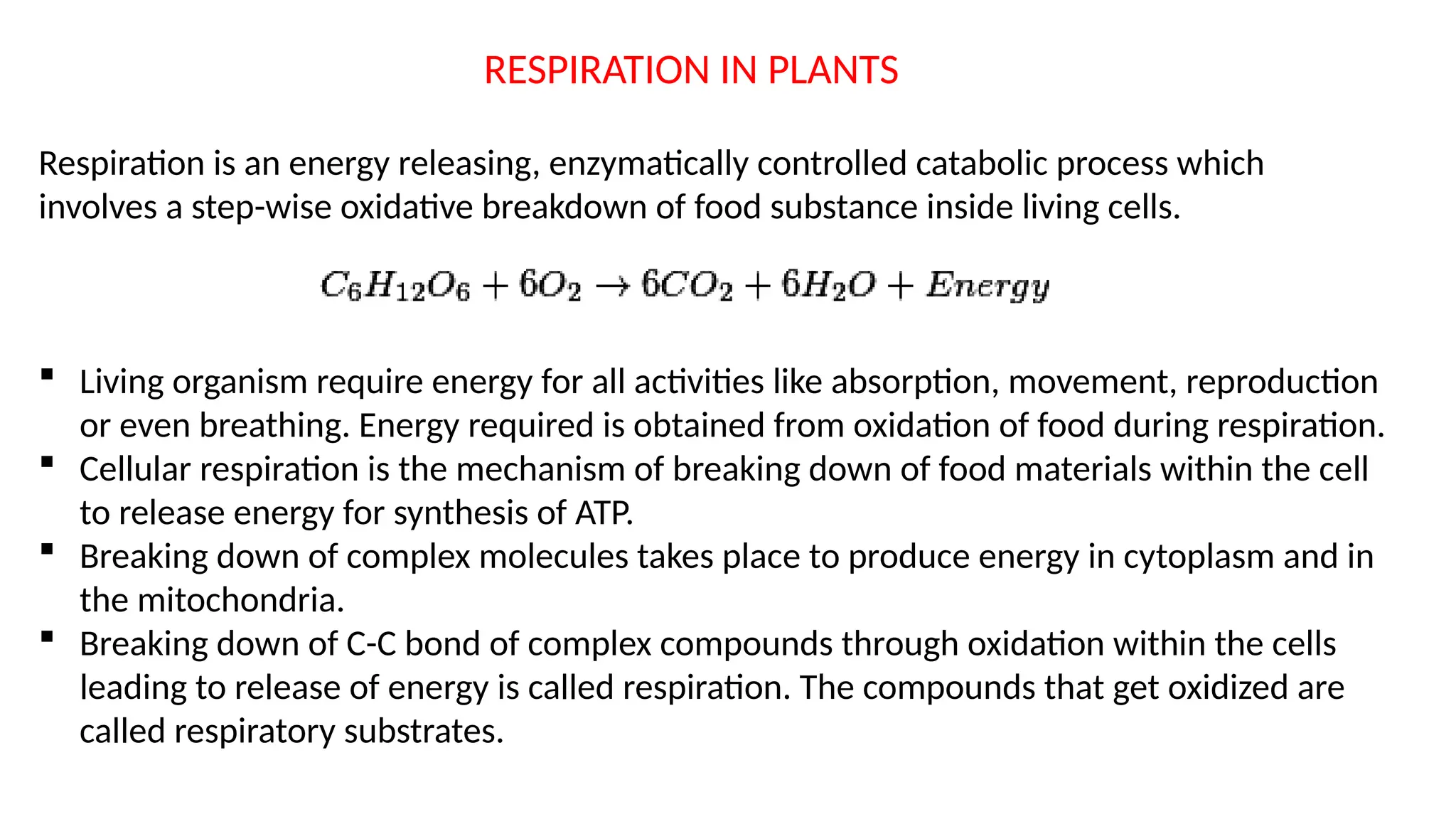
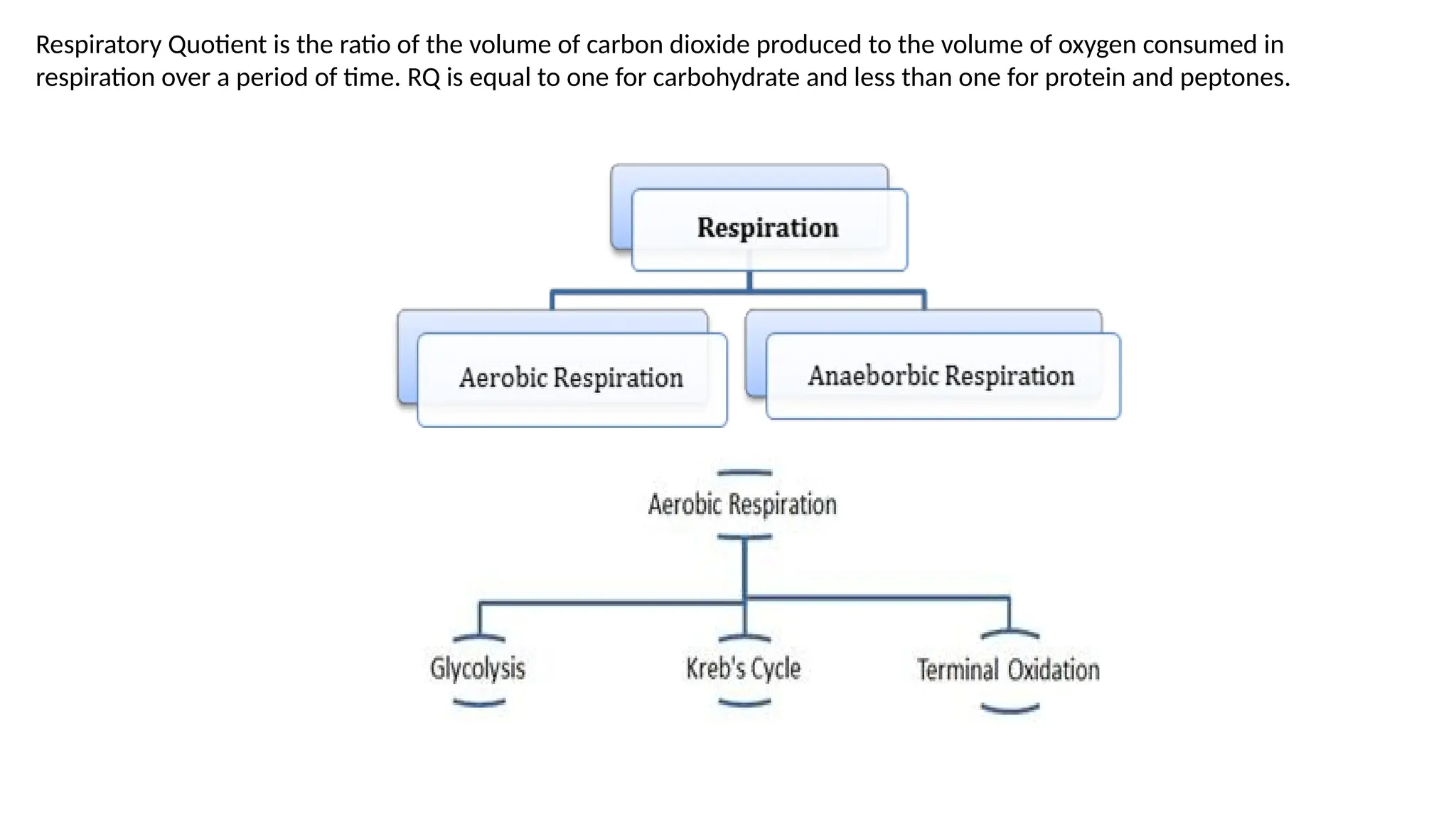
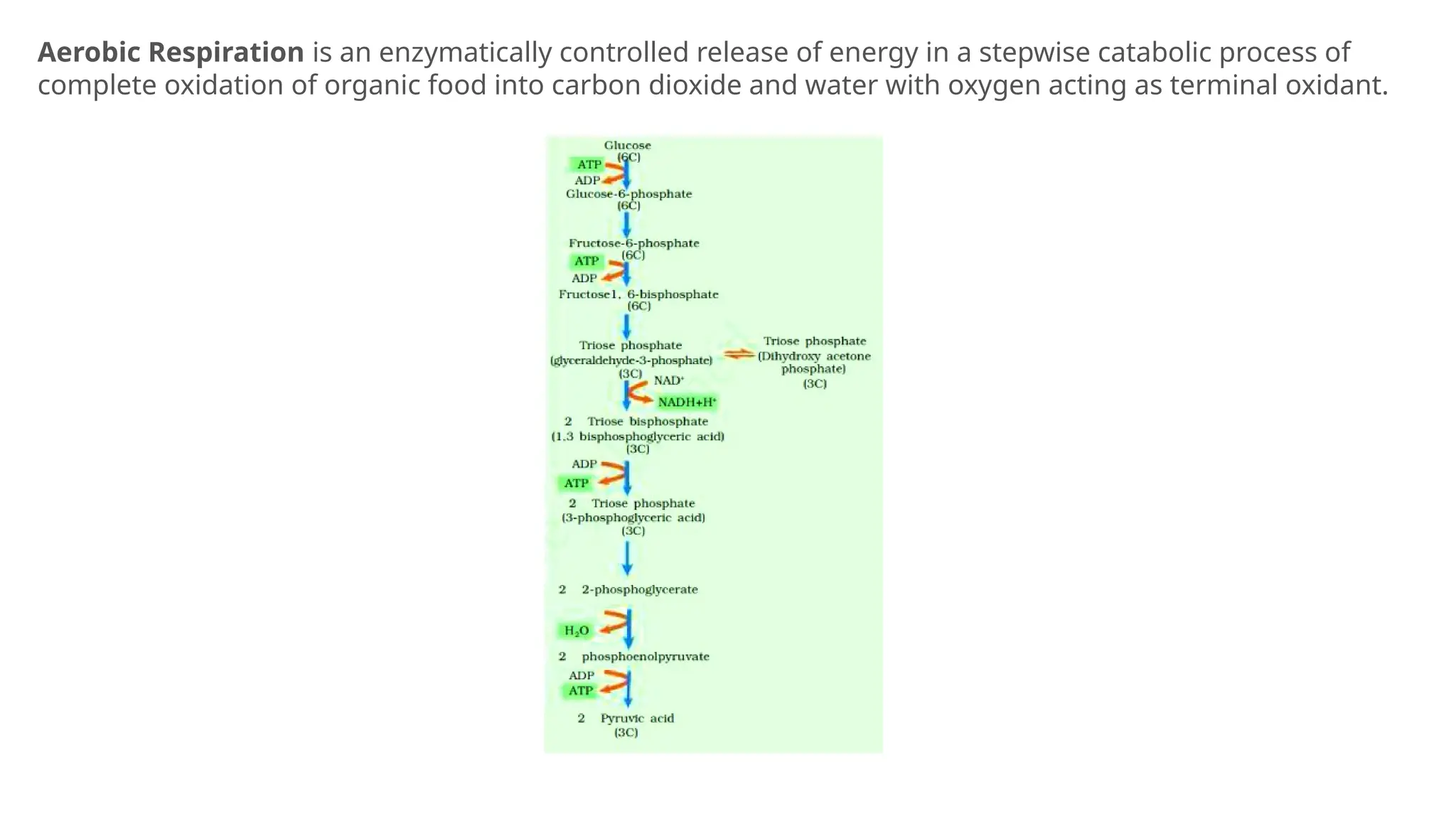
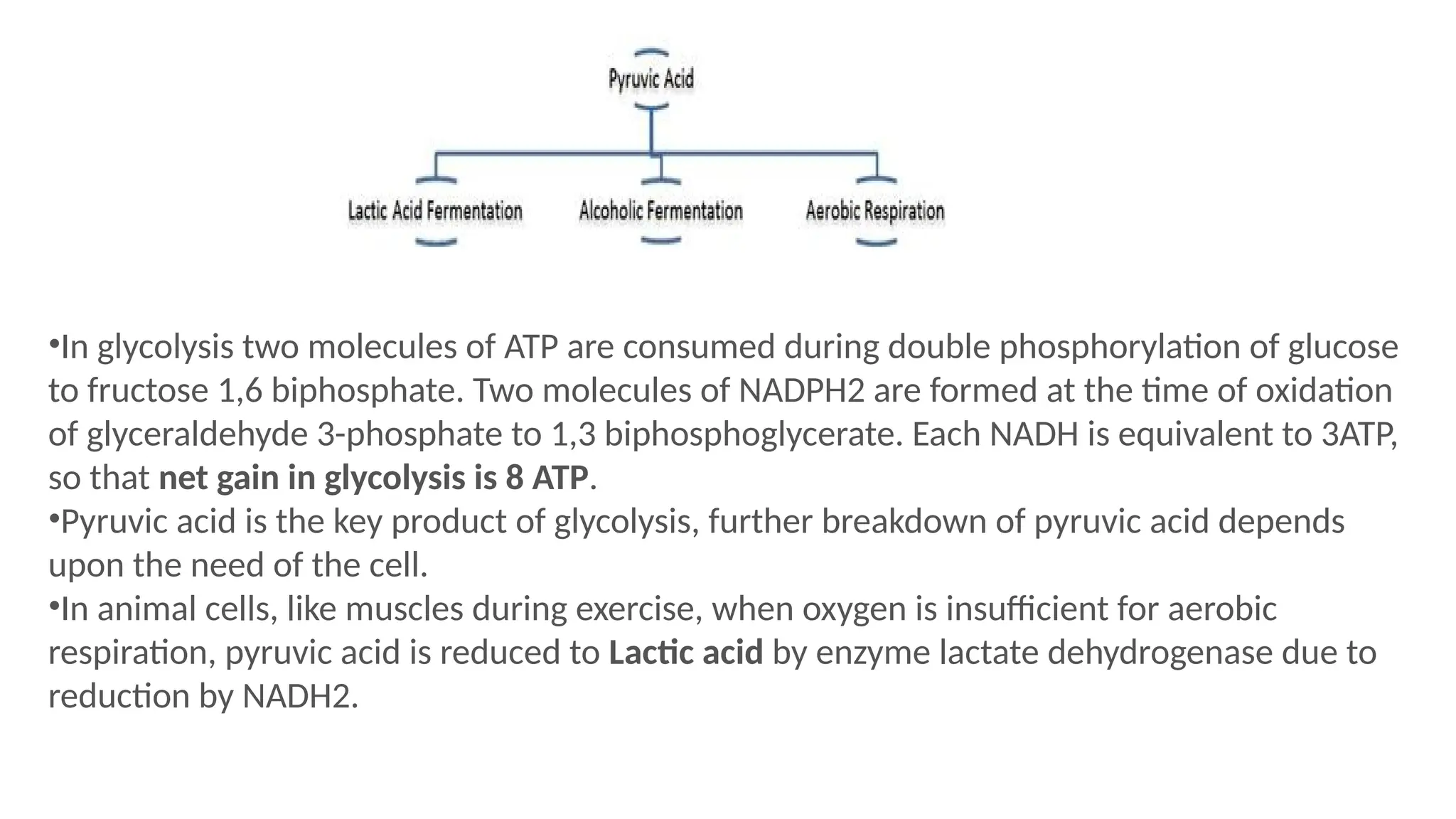
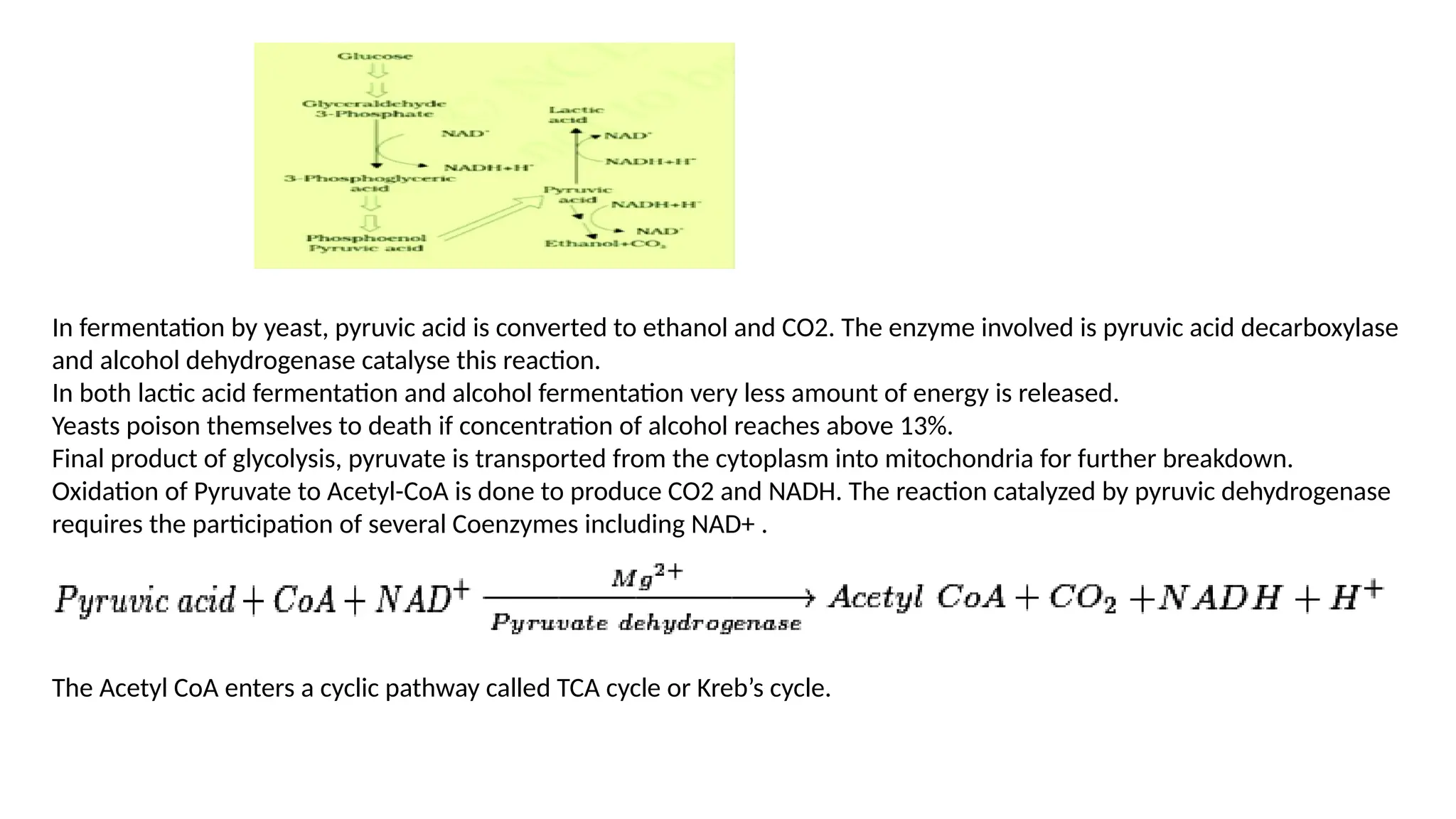
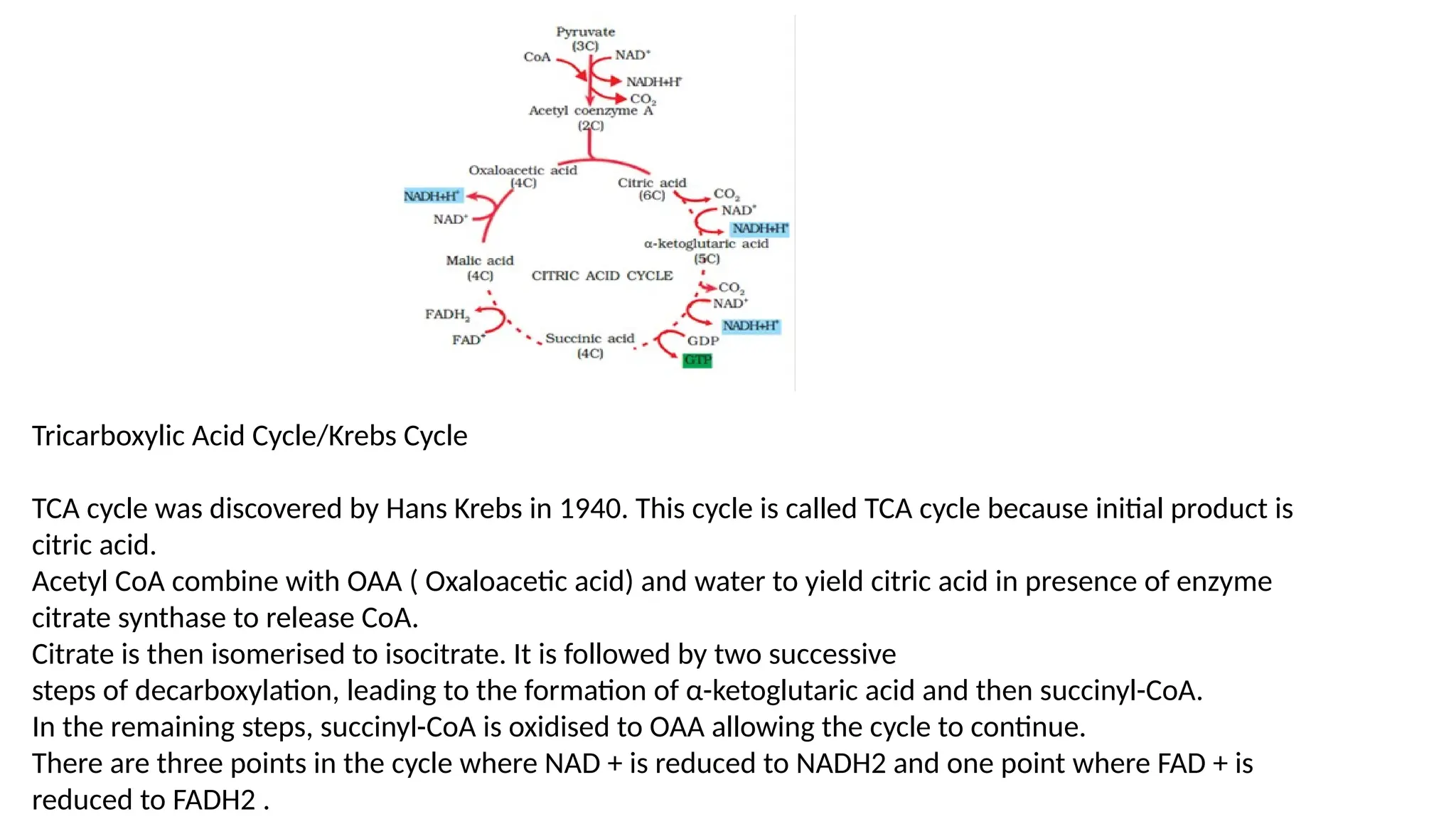
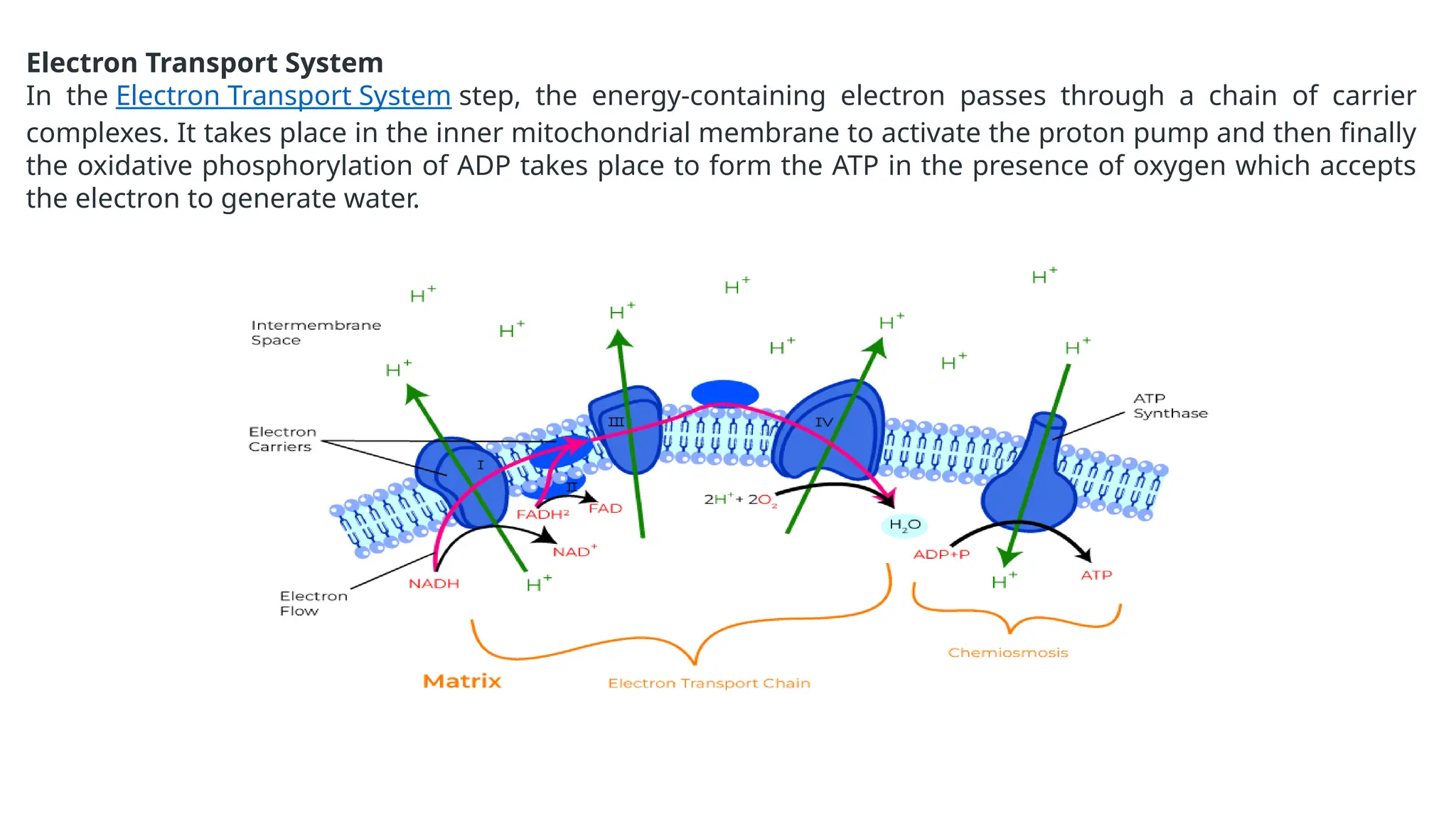
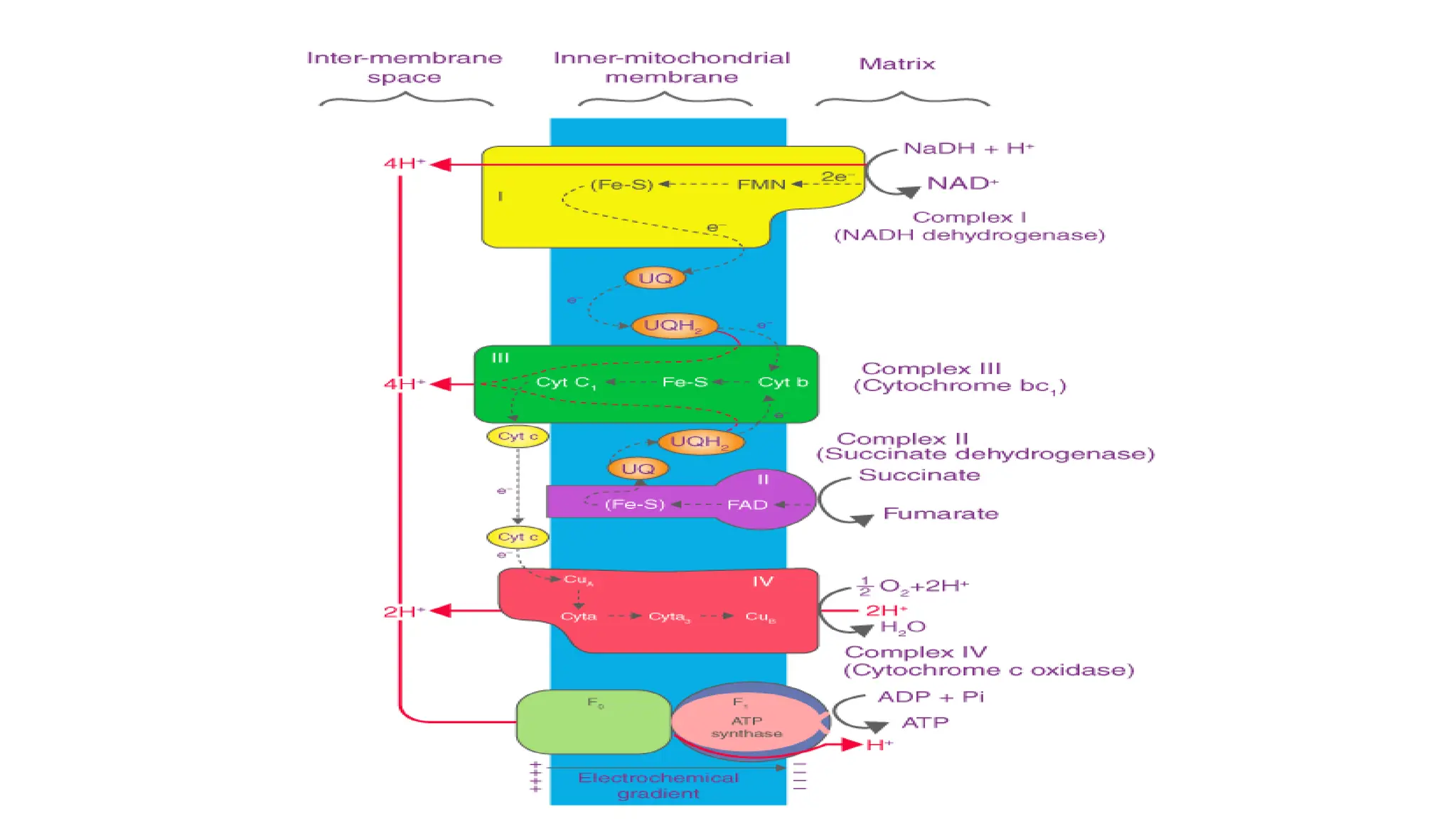
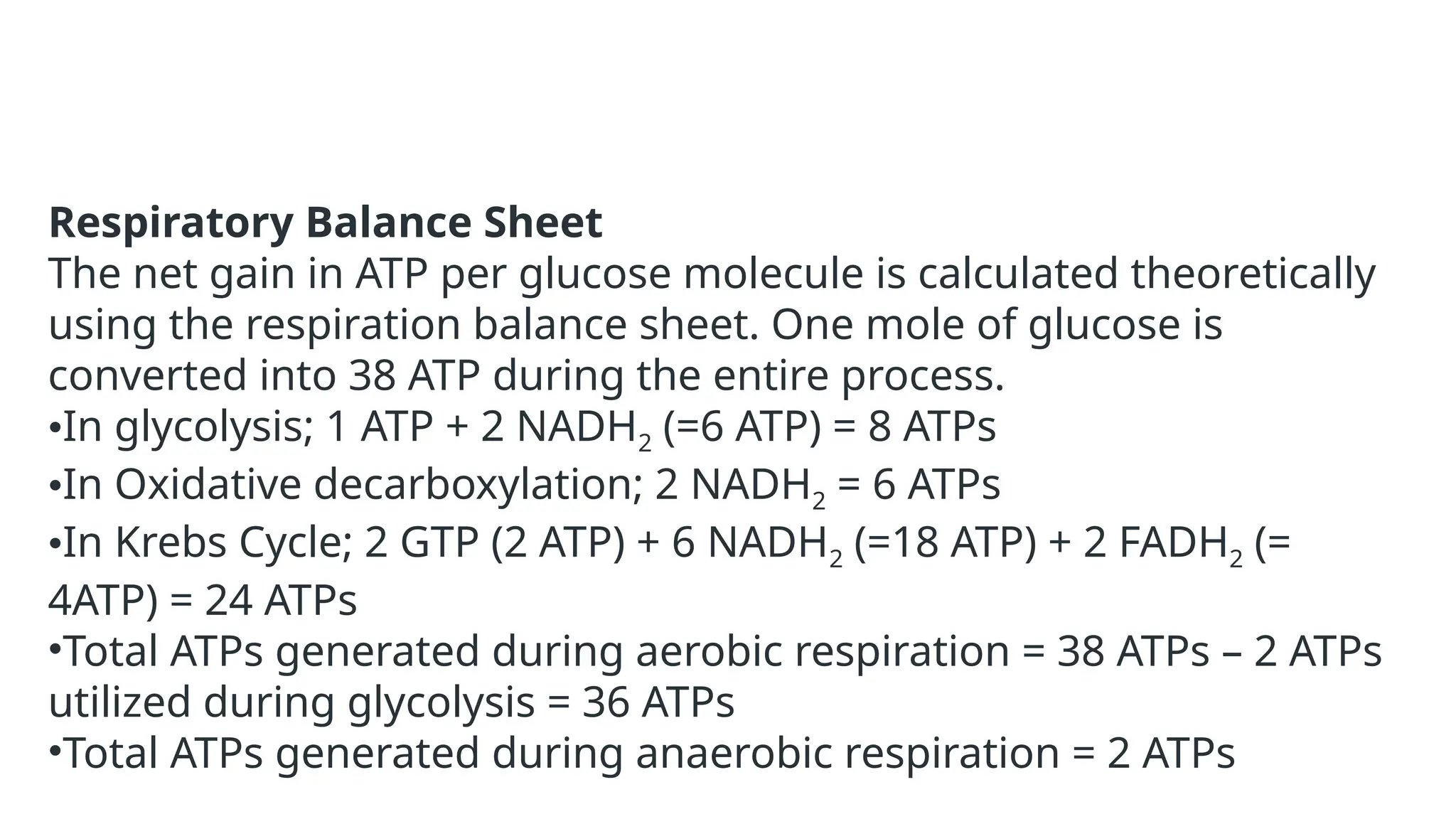
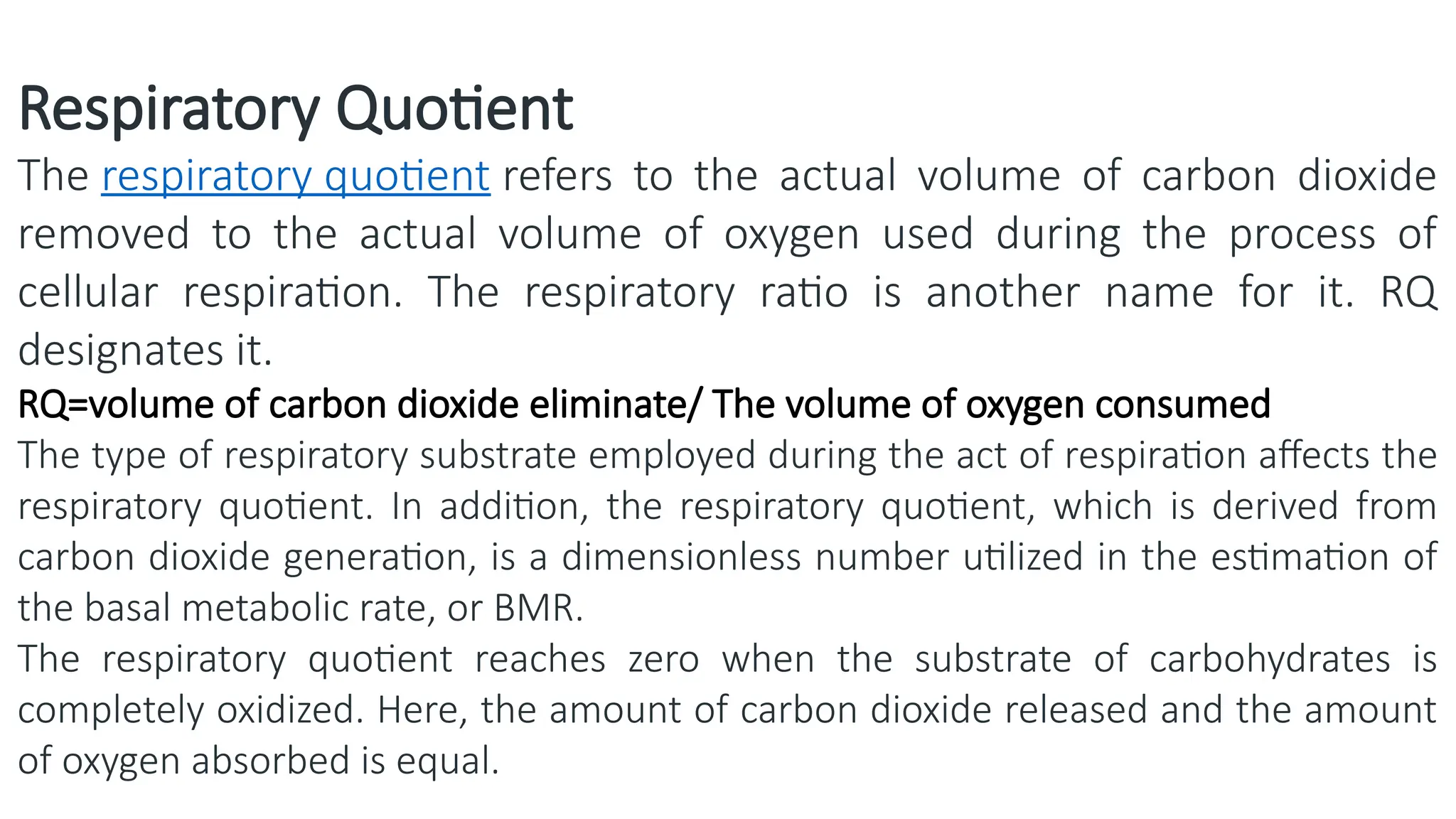
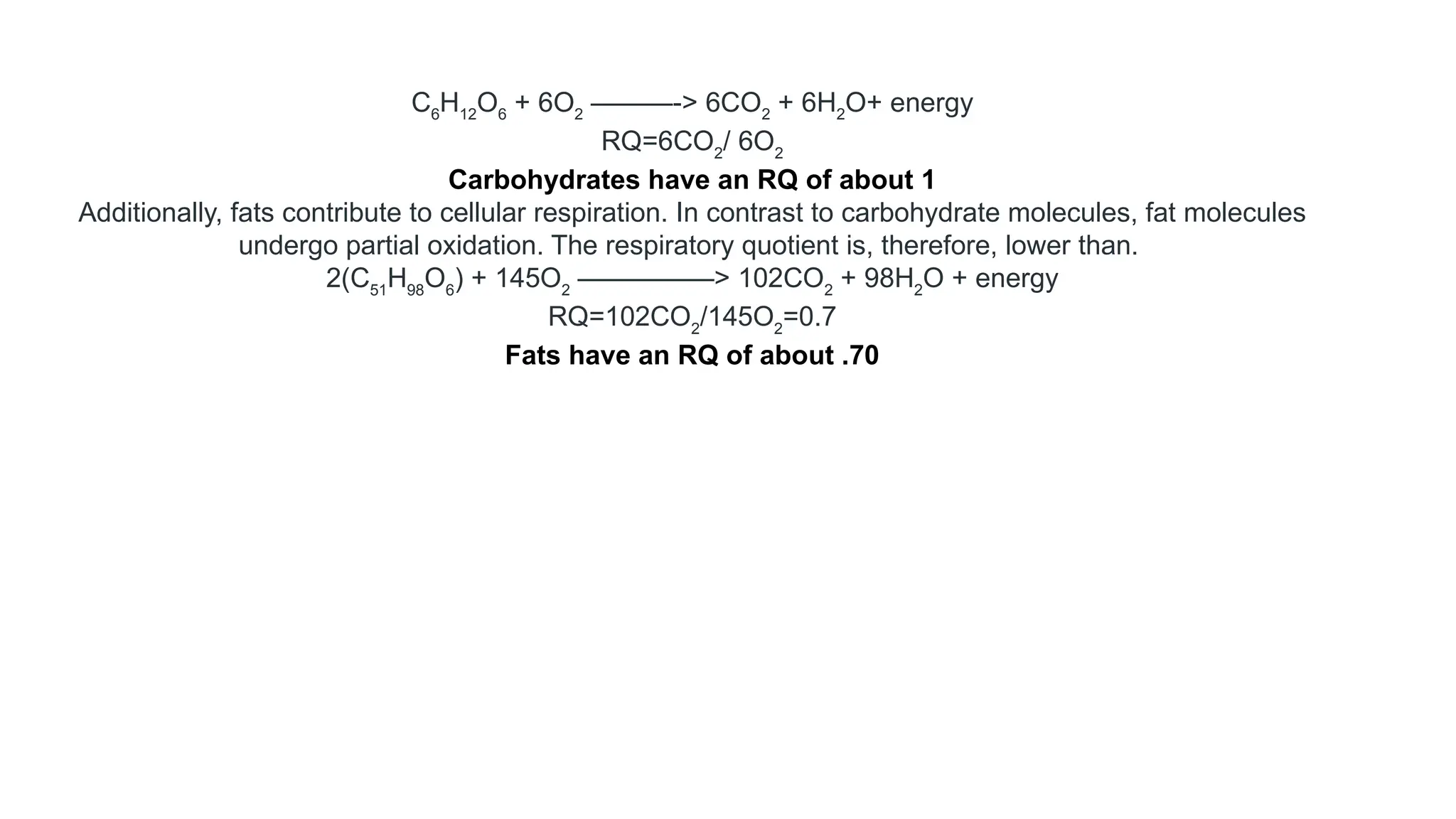
La respiración es un proceso catabólico controlado enzimáticamente que descompone sustancias alimenticias dentro de las células vivas para liberar energía, principalmente en forma de ATP. Este proceso incluye la glucólisis, el ciclo de Krebs y la cadena de transporte de electrones, donde el oxígeno es esencial y se producen dióxido de carbono y agua como subproductos. El cociente respiratorio mide la relación entre el dióxido de carbono producido y el oxígeno consumido durante la respiración celular, con valores que varían entre diferentes sustratos metabólicos.