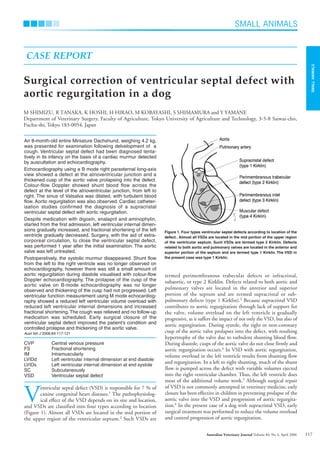
This document describes a case study of a Miniature Dachshund that was diagnosed with a ventricular septal defect (VSD) and aortic regurgitation. Echocardiography revealed a defect between the ventricles and a thickened aortic valve prolapsing into the defect. Cardiac catheterization confirmed a supracristal VSD with aortic regurgitation. Despite medication, the dog's left ventricular dimensions worsened over time. The dog ultimately underwent surgery to close the VSD using cardiopulmonary bypass, which improved the condition and controlled further valve deterioration.