This document provides an overview of renal failure, including:
- Classification of acute and chronic renal failure
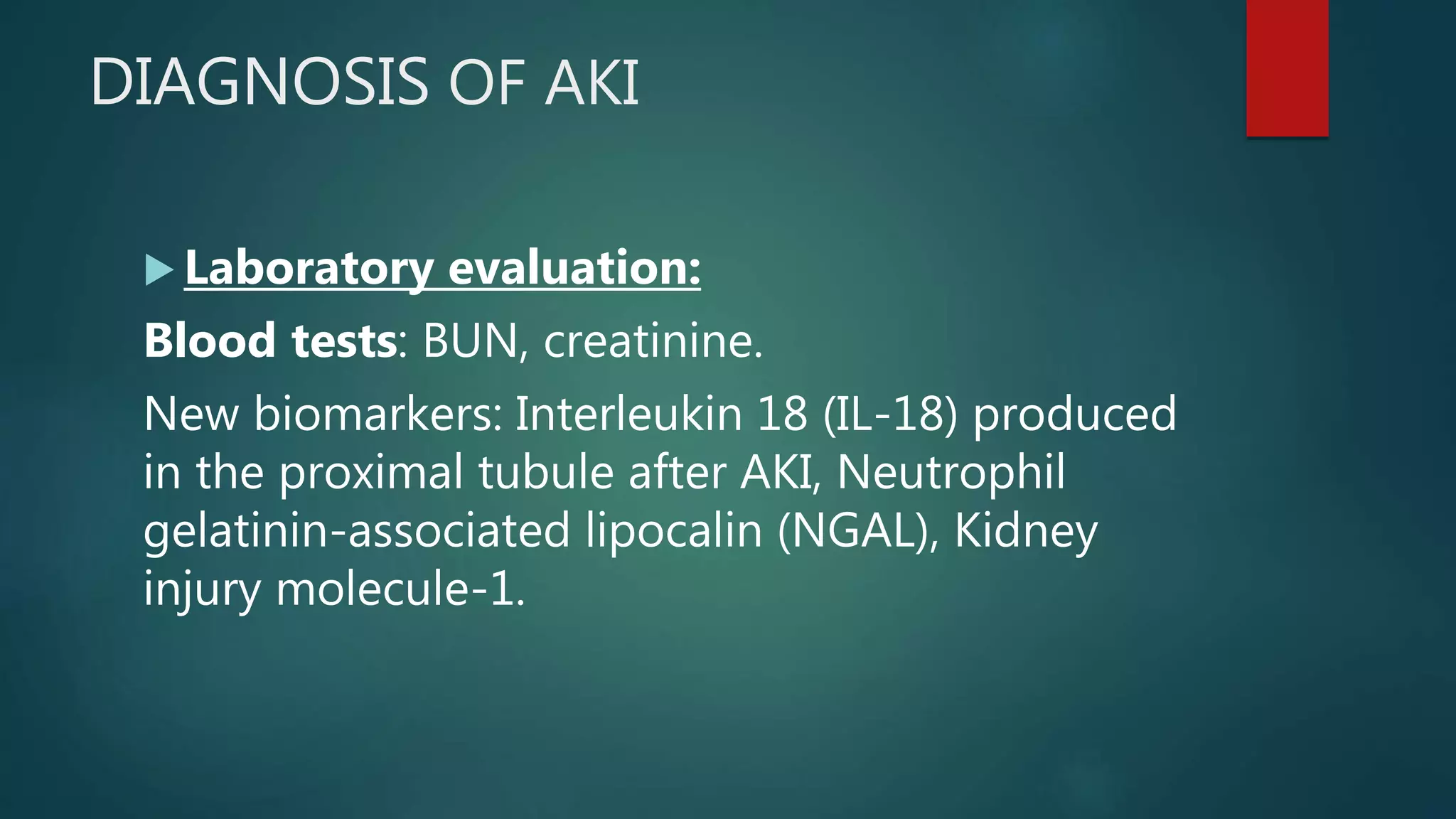
- Definitions, causes, pathophysiology and treatment of acute kidney injury (AKI) and chronic kidney disease (CKD)
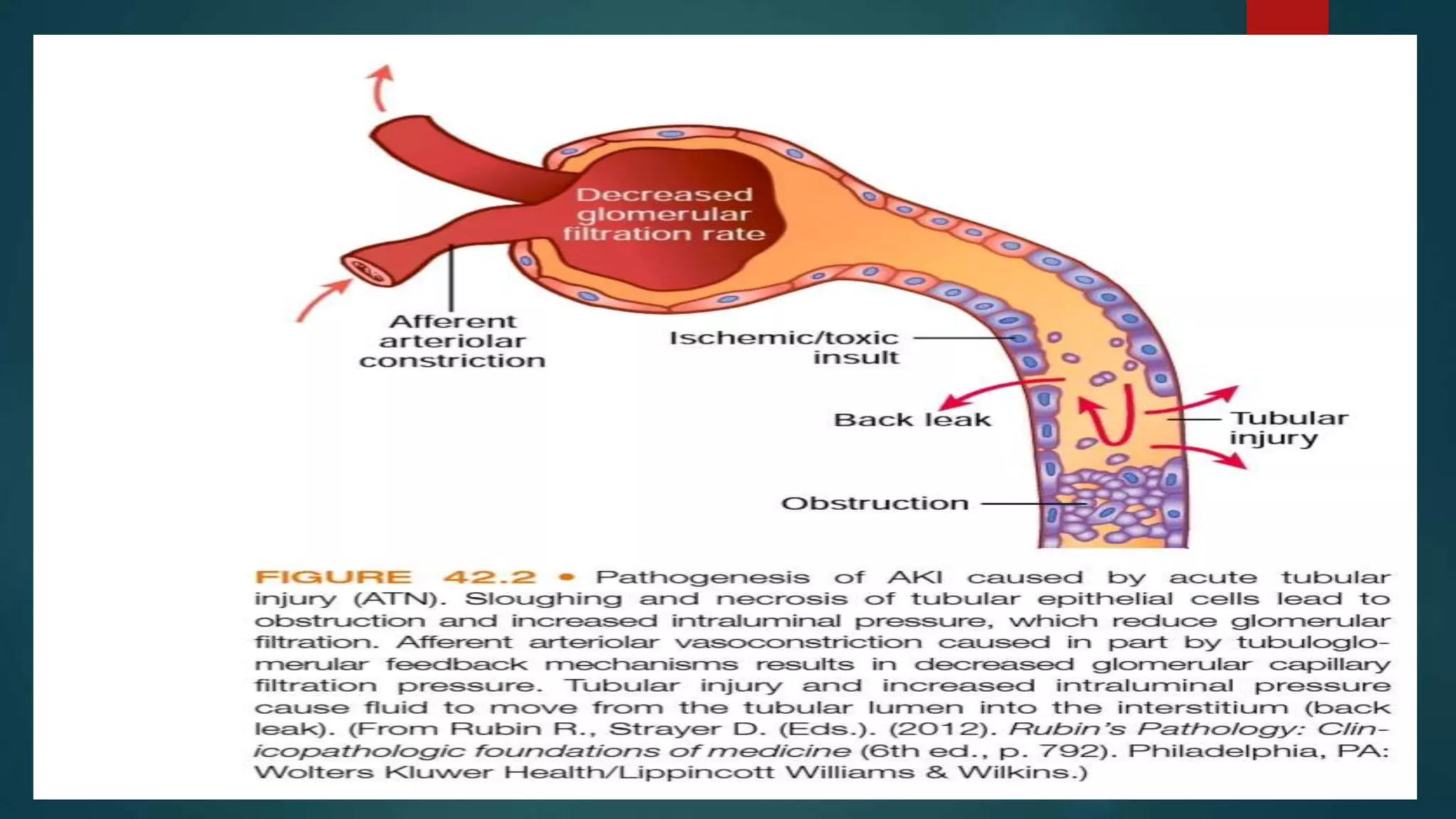
- Prerenal, intrarenal and postrenal causes of AKI
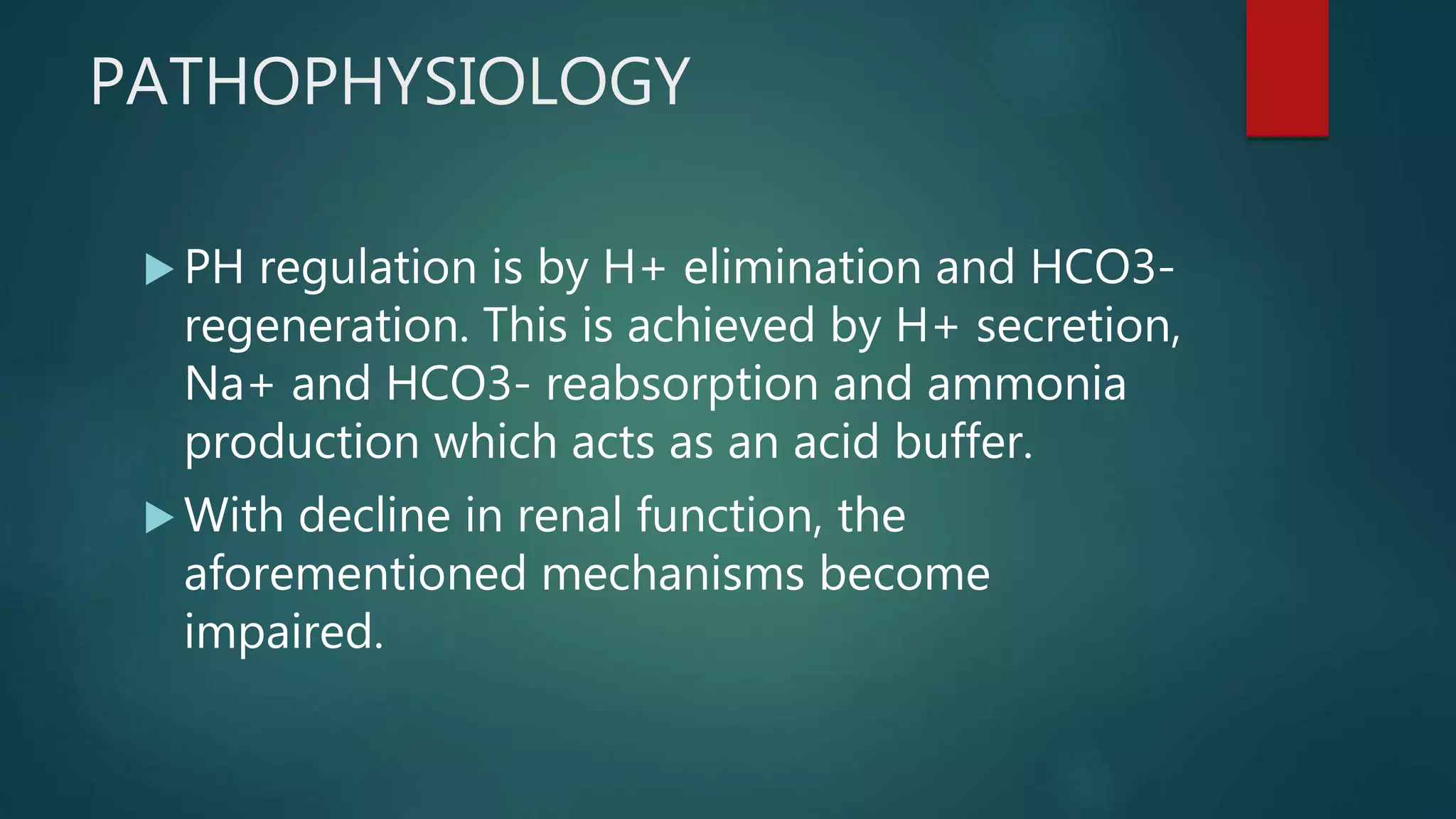
- Clinical manifestations and pathophysiology of CKD including accumulation of waste, fluid and electrolyte disturbances, and calcium/phosphorus disorders
- Treatment focuses on slowing CKD progression, managing complications, and dialysis or transplant for advanced disease.