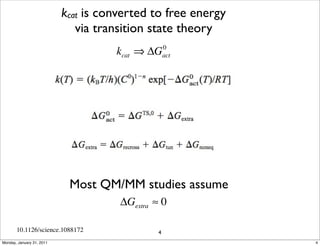
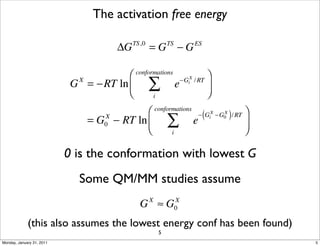
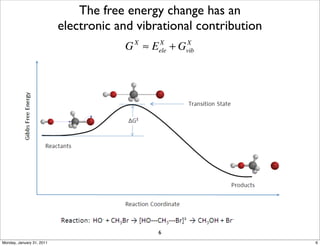
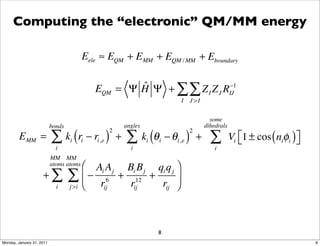
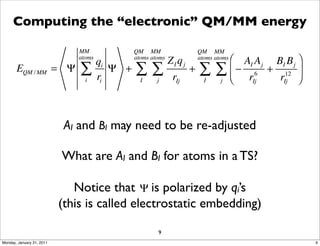
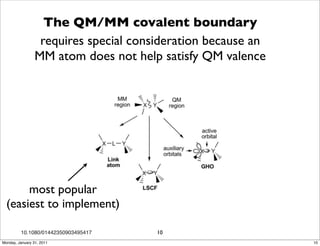
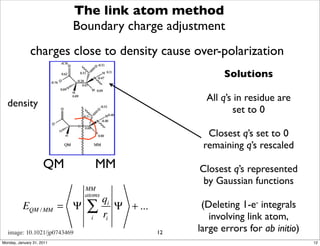
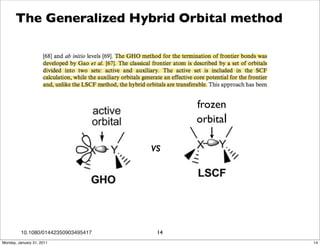
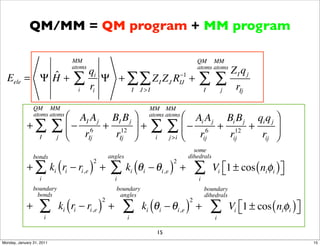
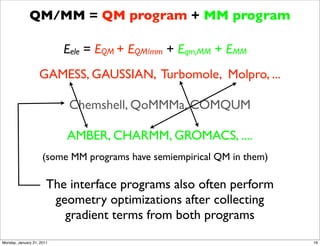
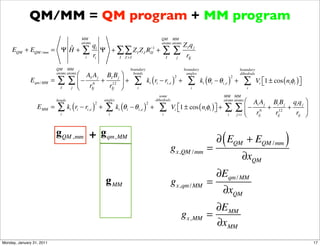
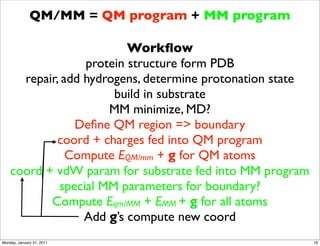
1) The document discusses the use of quantum mechanics/molecular mechanics (QM/MM) methods to study reaction mechanisms in enzymes. It outlines some of the challenges in applying these methods, including accurately computing energies and free energy contributions and finding transition states.
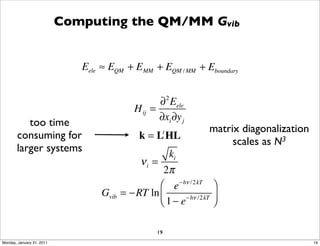
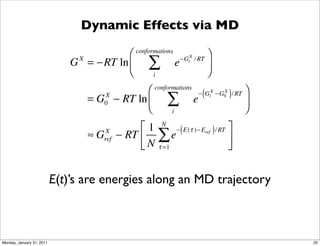
2) Key aspects of QM/MM calculations that are discussed include separating the electronic and vibrational contributions to free energy, treating the boundary between the QM and MM regions, and using molecular dynamics simulations to account for dynamic effects.
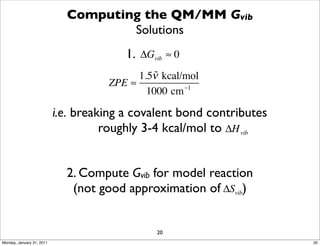
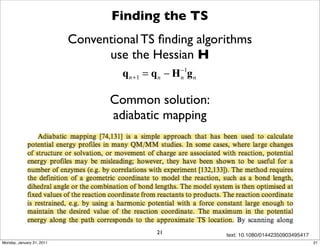
3) The document provides an overview of common approaches to addressing issues like computing energies, incorporating vibrational contributions, optimizing transition states, and representing the QM/MM boundary region in the calculations.
![Measured: rate [P]/s
Rate => kcat
10.1126/science.1088172 3
Monday, January 31, 2011 3](https://image.slidesharecdn.com/wk1-110204035717-phpapp02/85/QM-MM-Background-3-320.jpg)