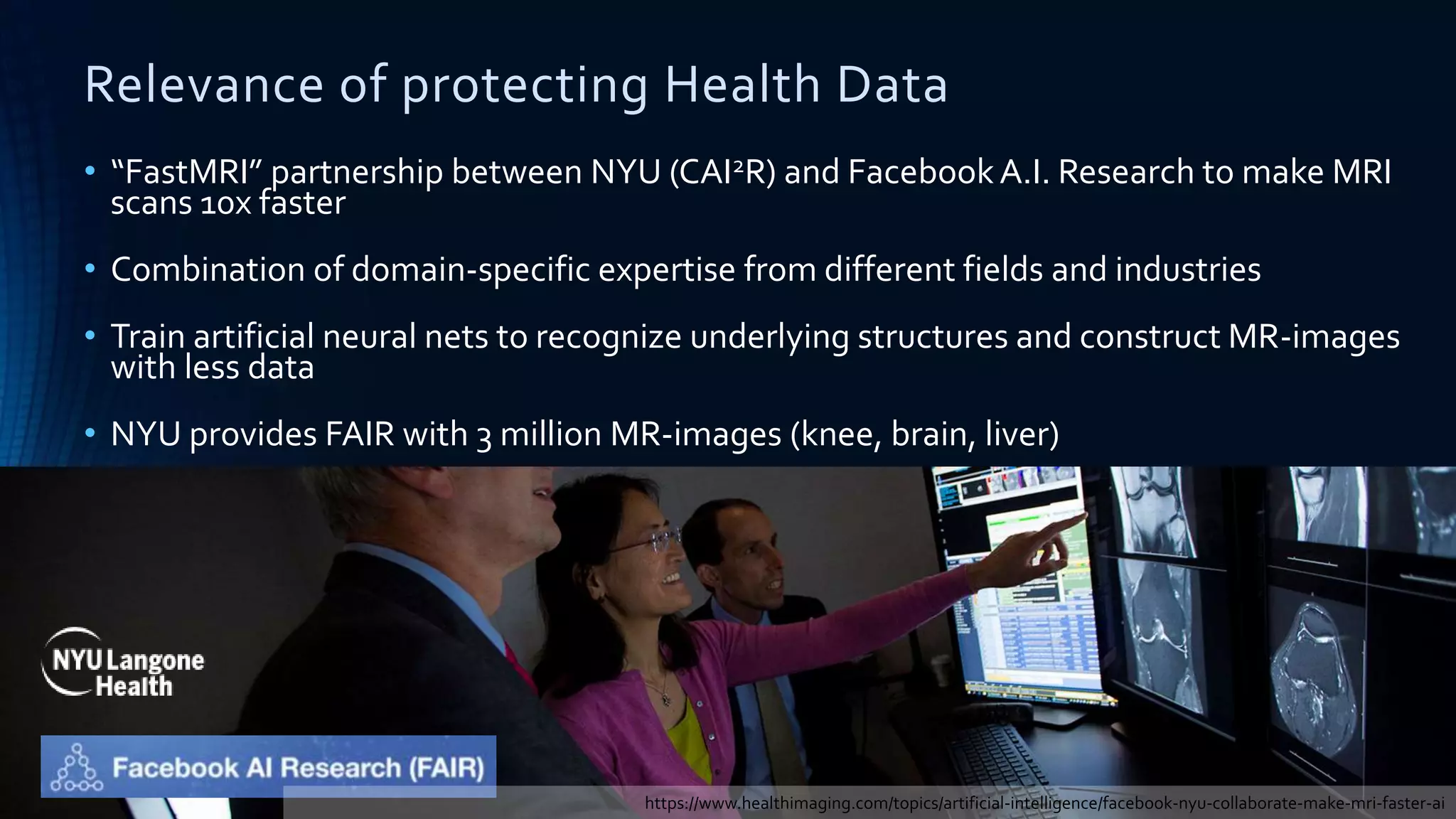
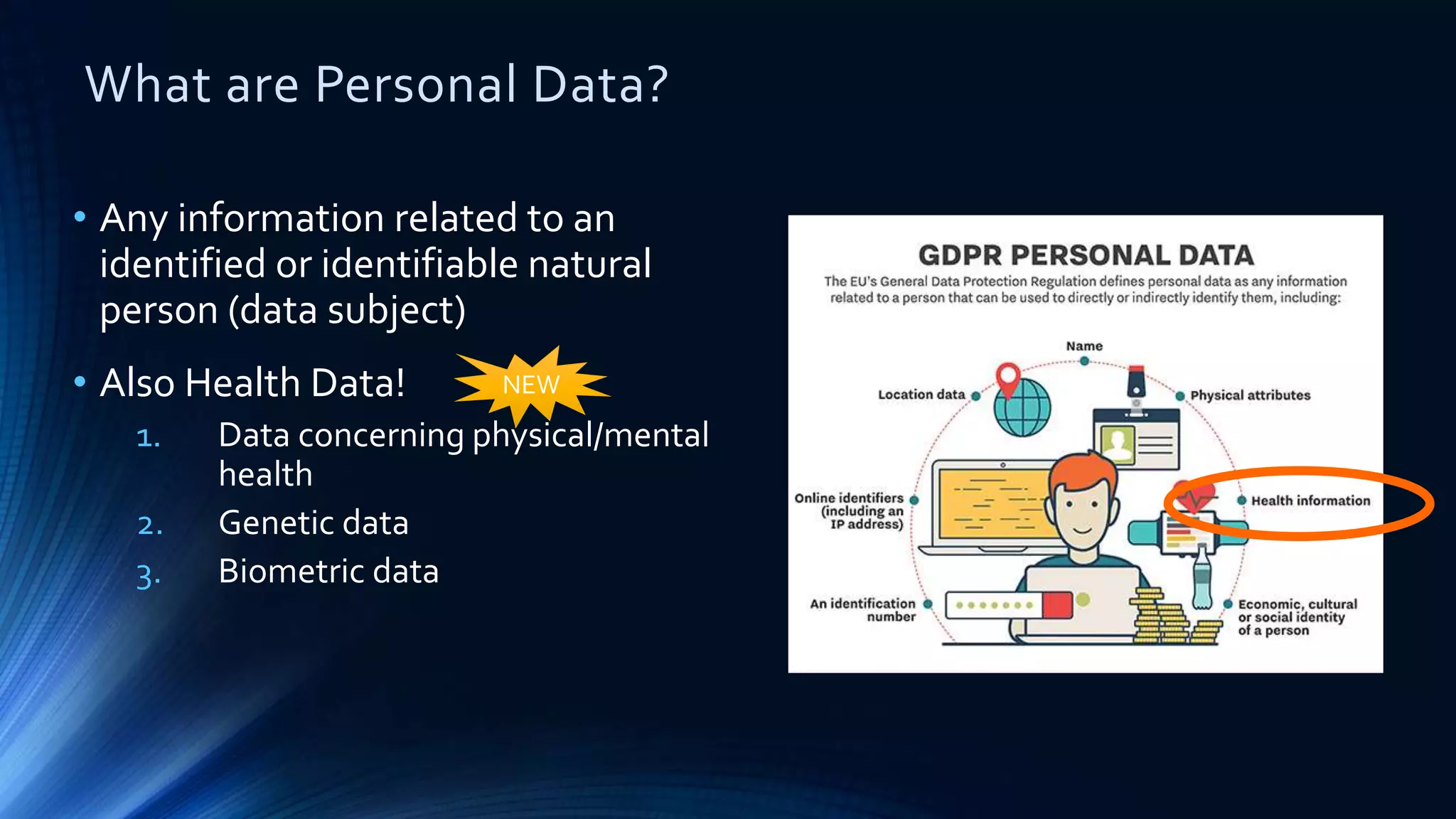
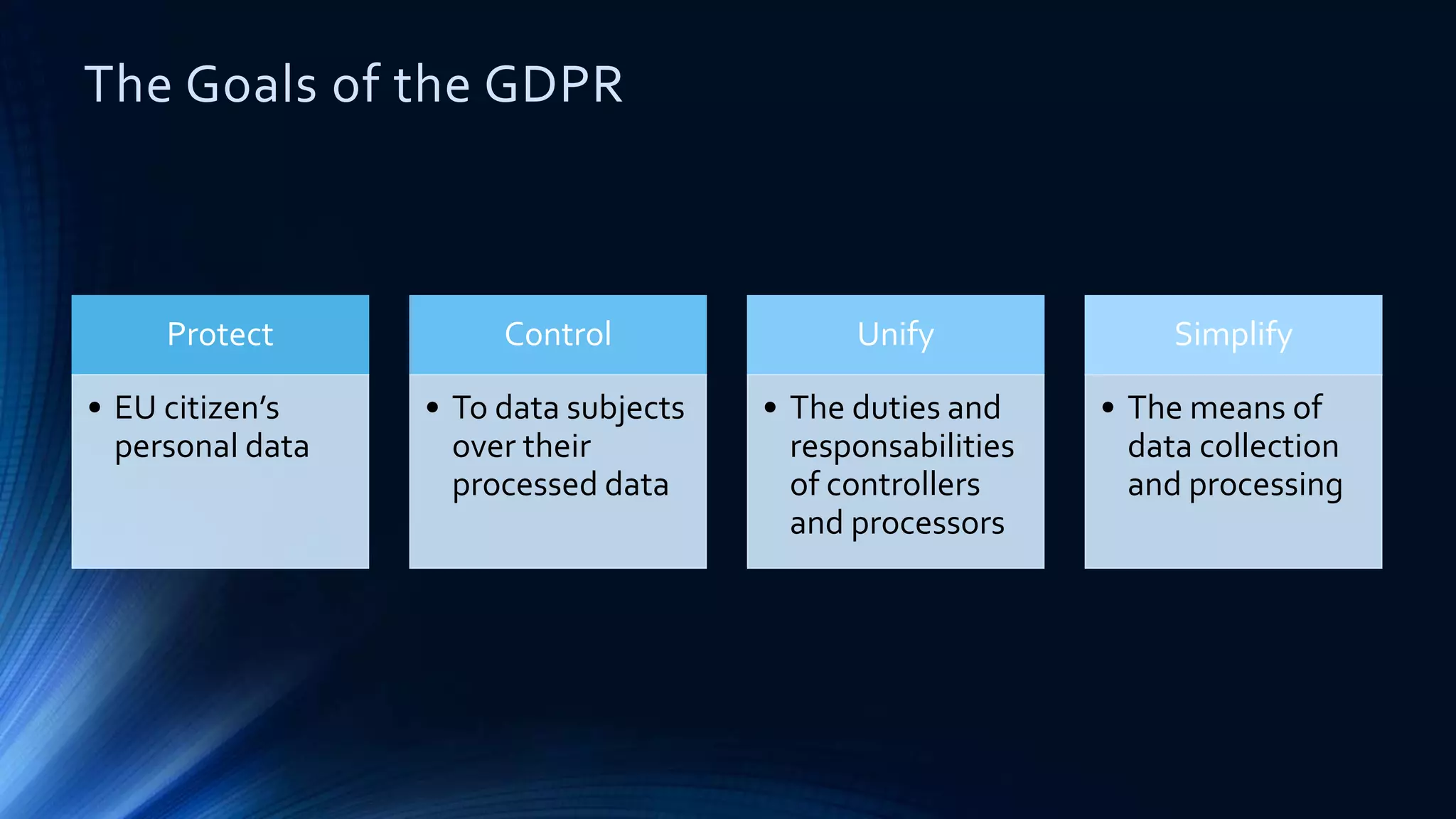
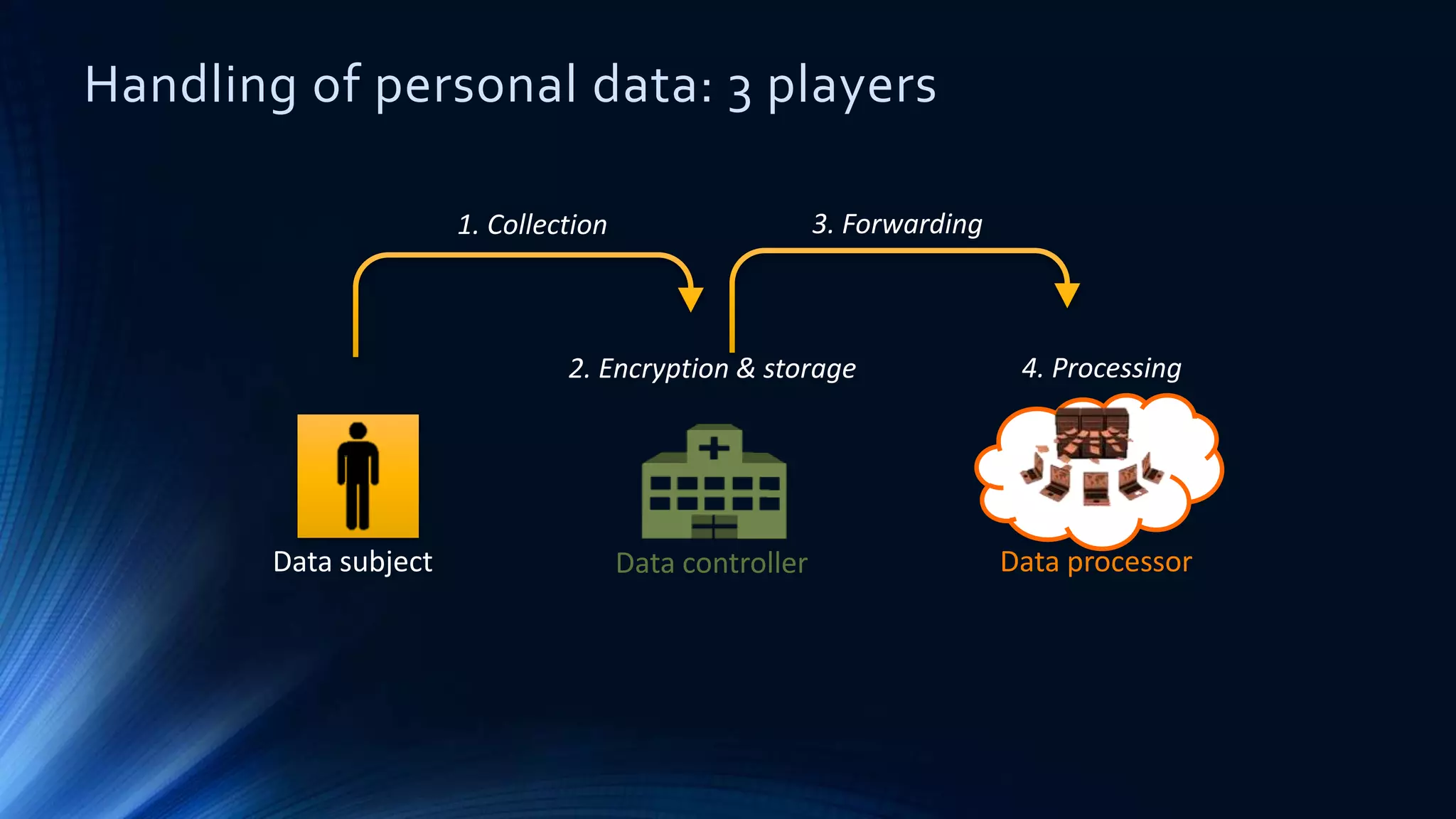
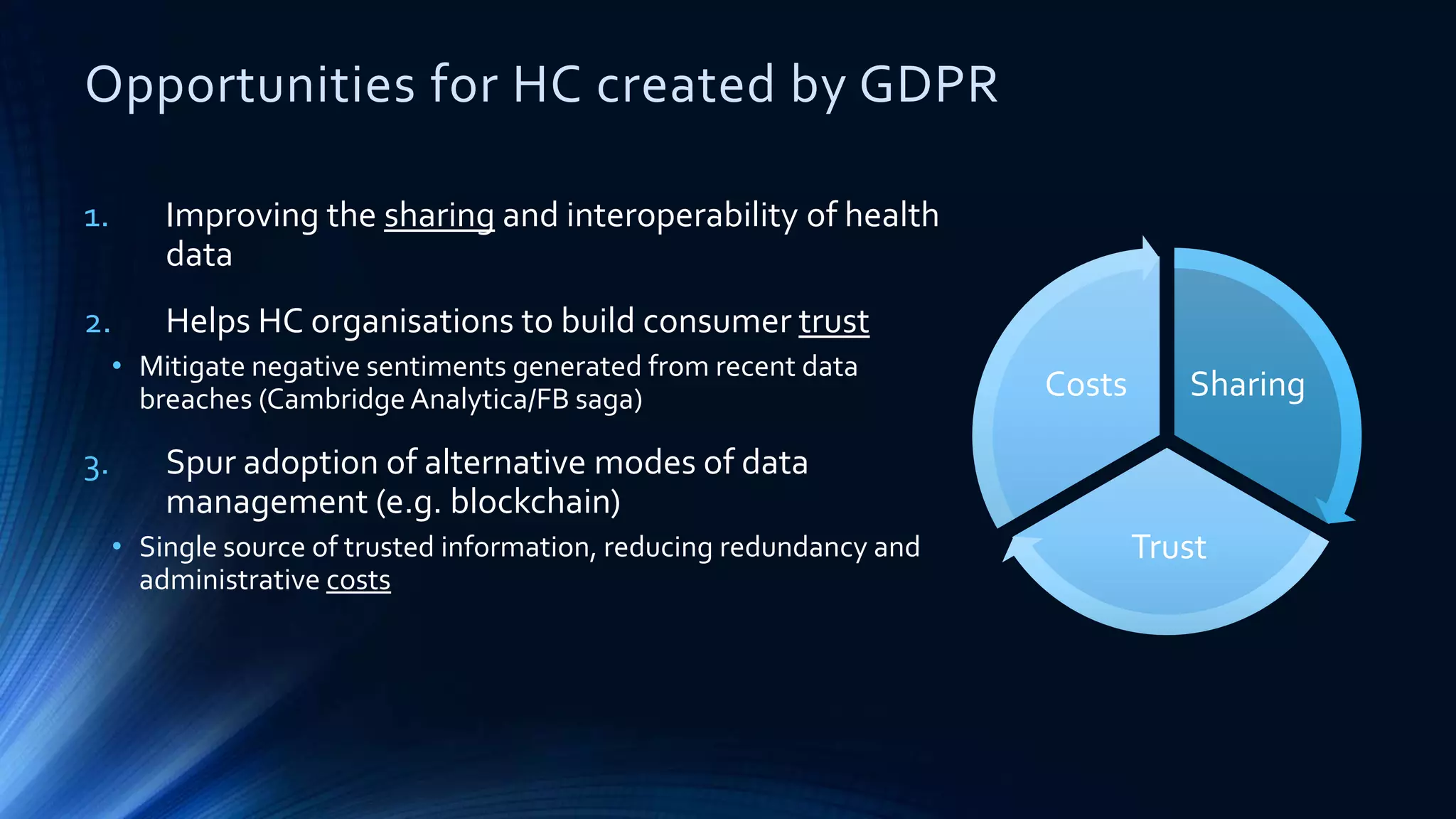
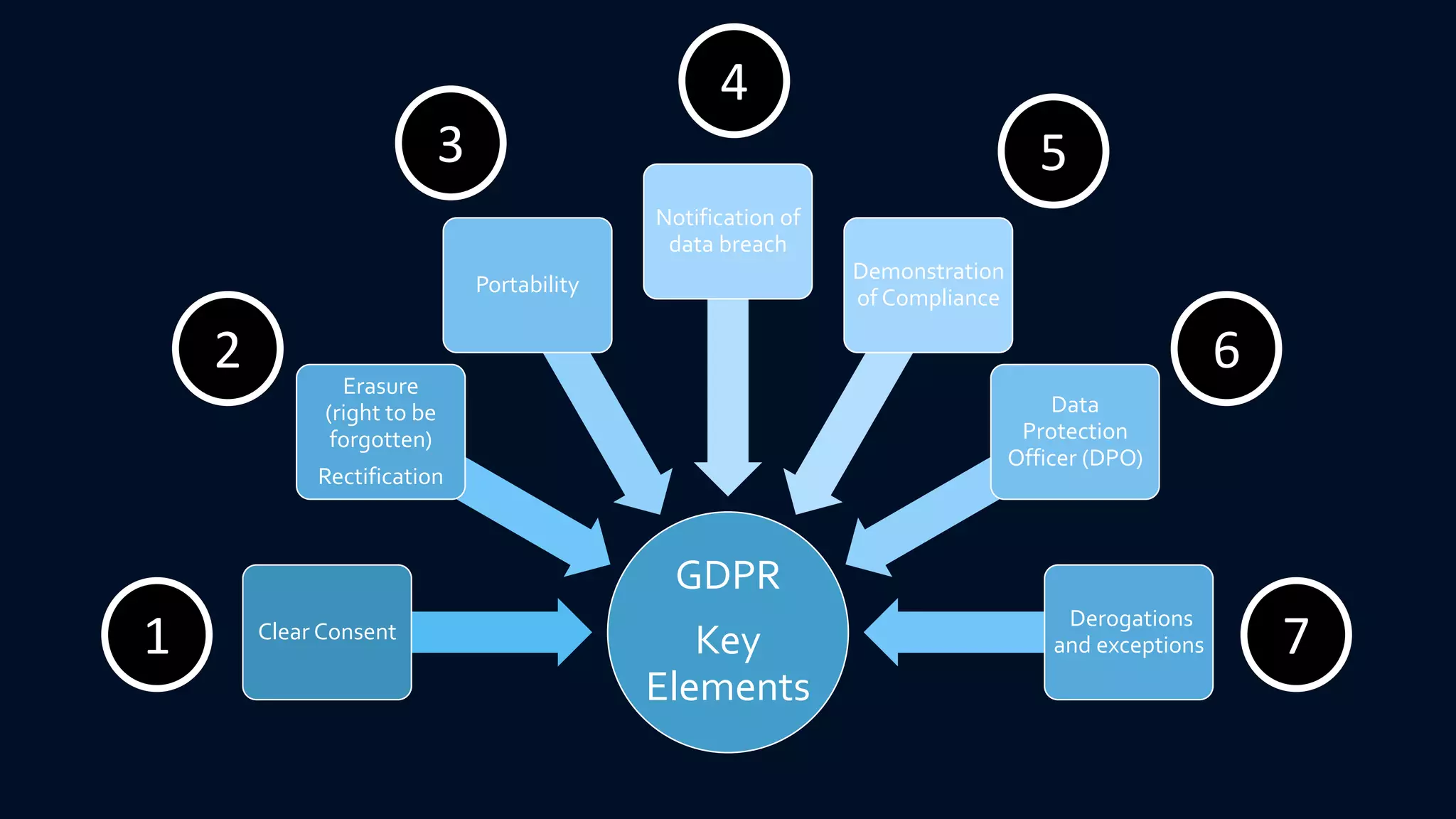
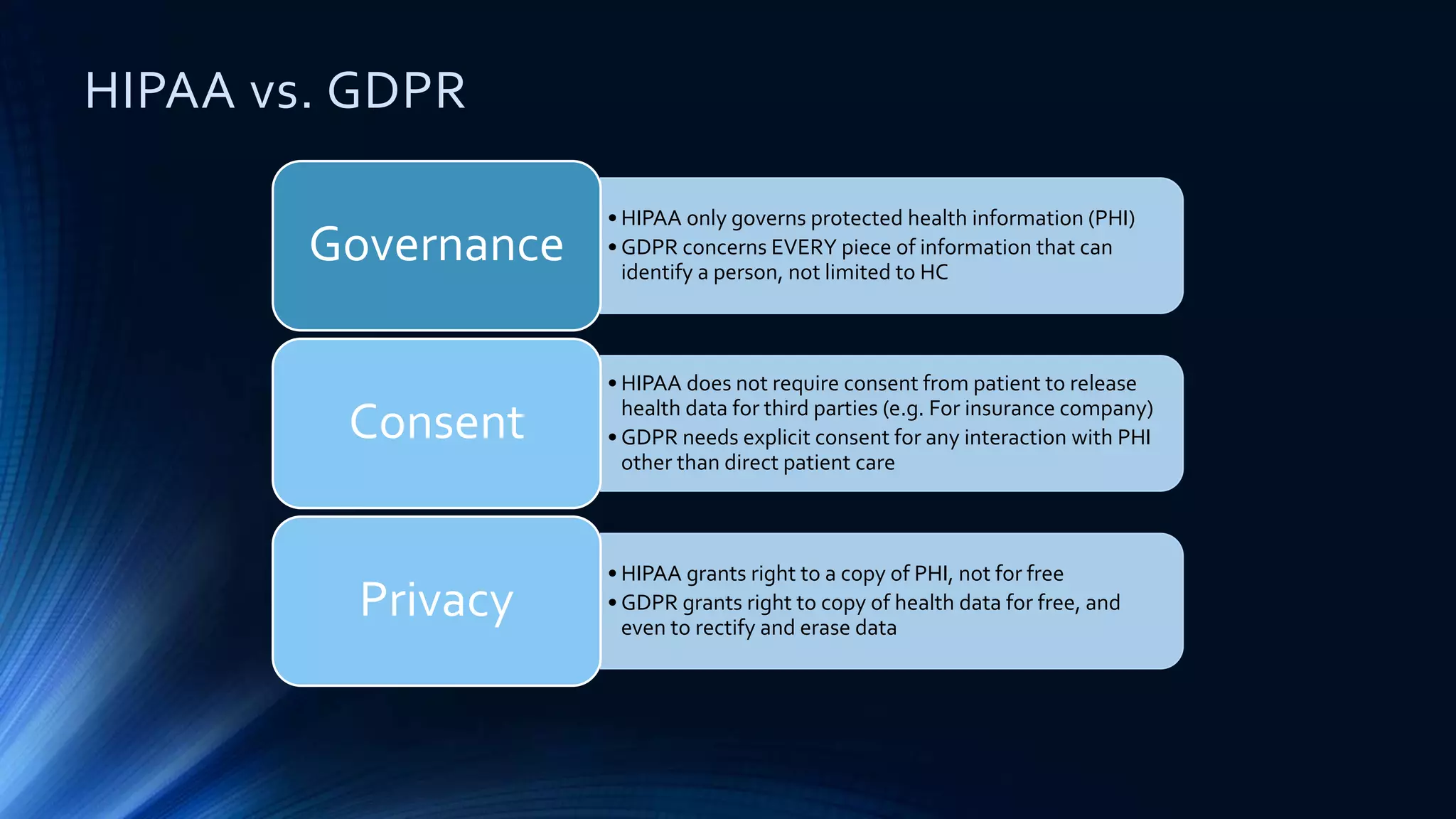
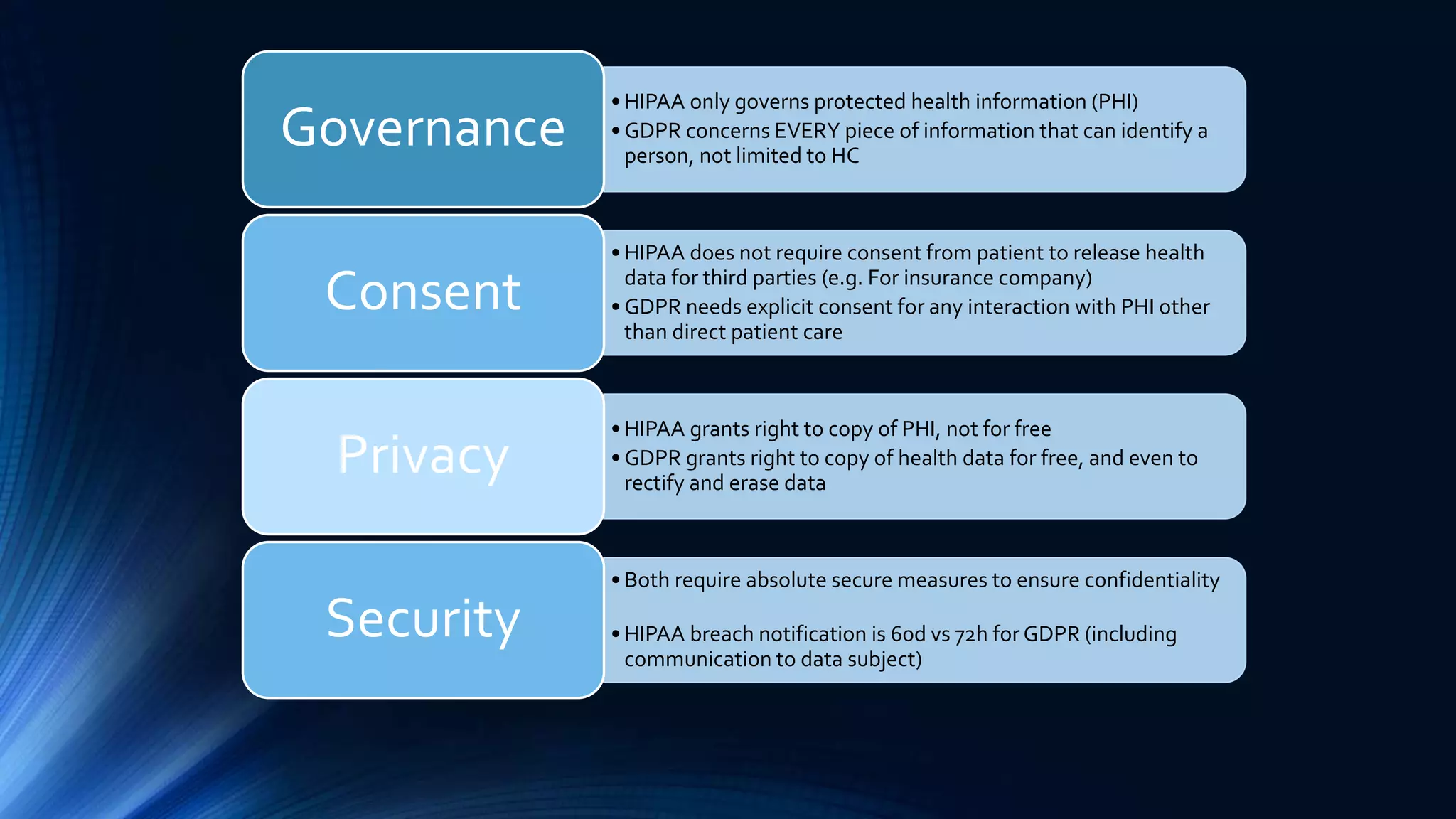
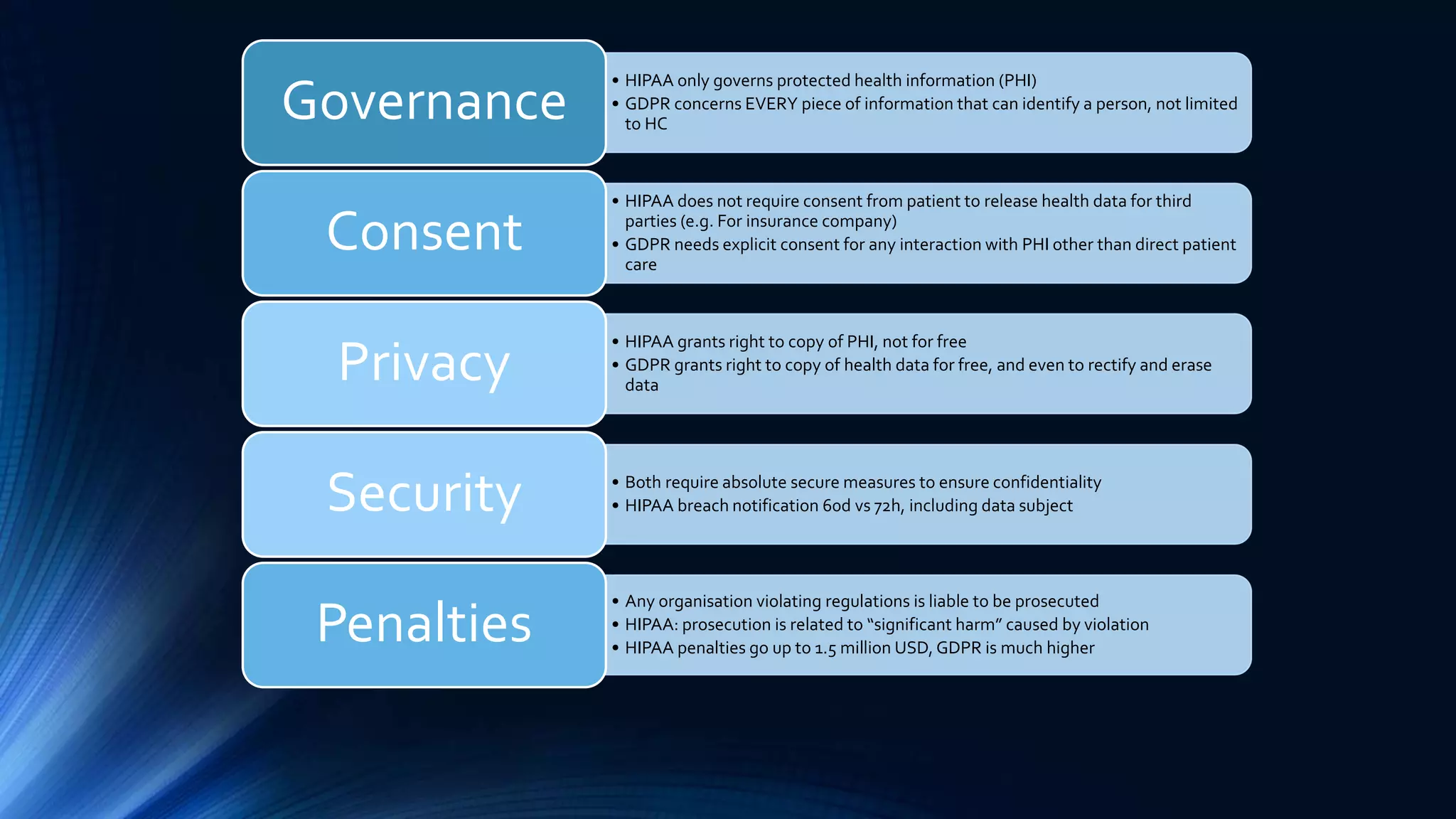
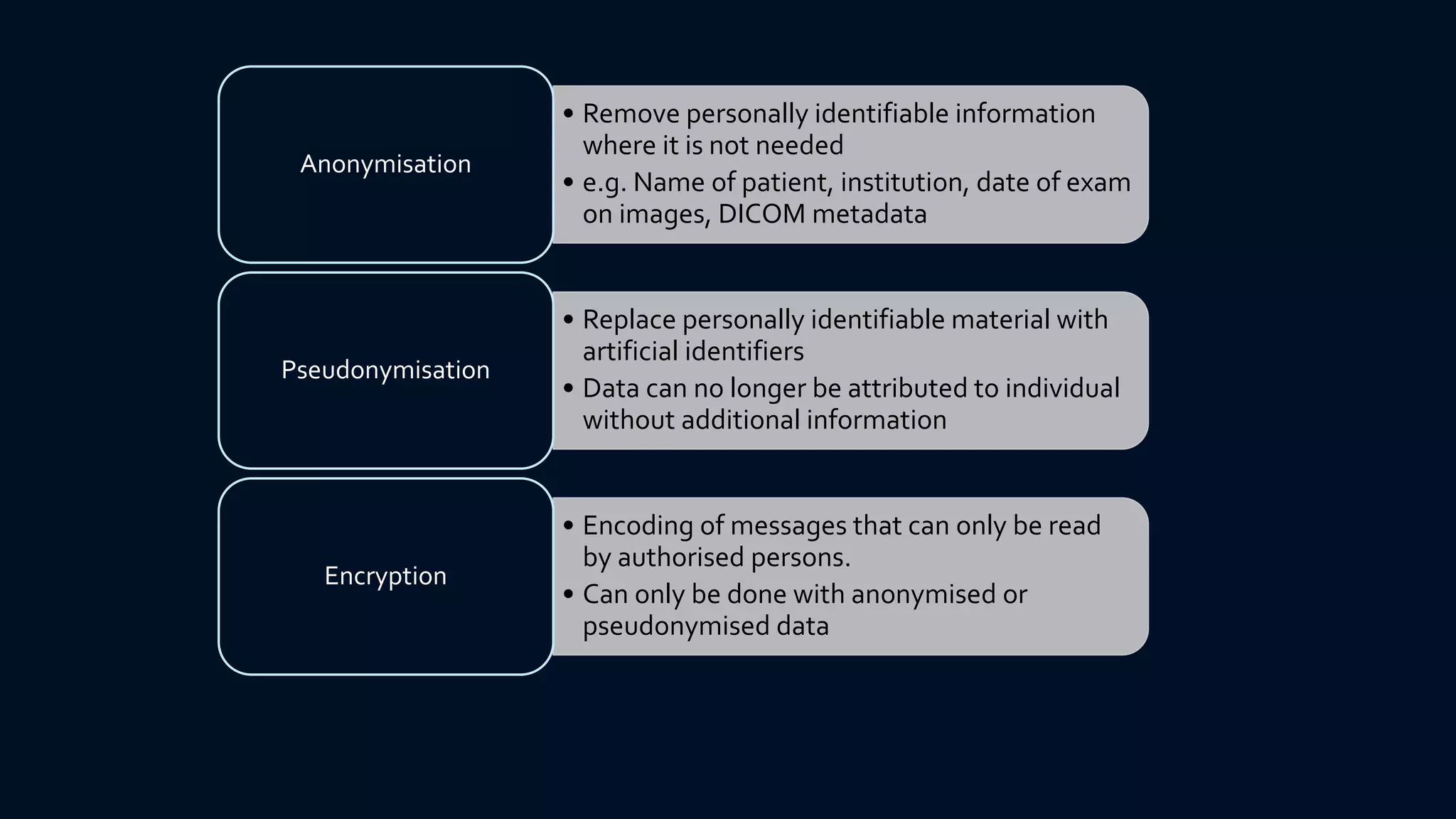
The document compares the protection of patient data in the EU and US, focusing on the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA). It highlights key differences between the two regulations, including consent requirements, data breach notifications, and penalties for non-compliance. The document also addresses ethical concerns related to the use of AI in healthcare and the complexities of obtaining consent for research purposes.