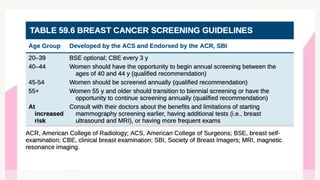
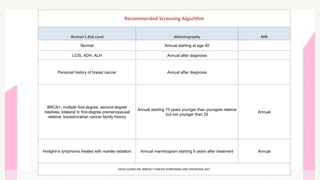
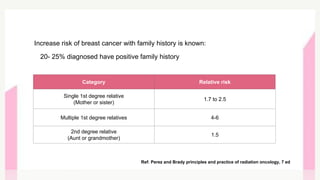
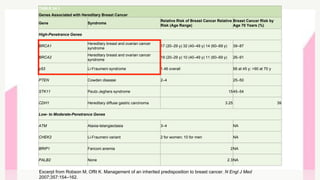
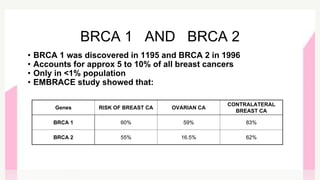
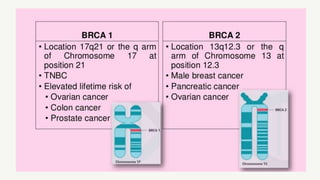
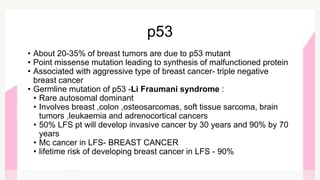
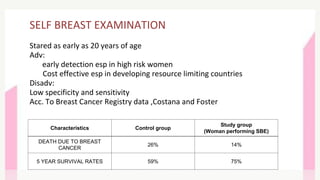
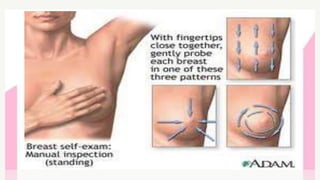
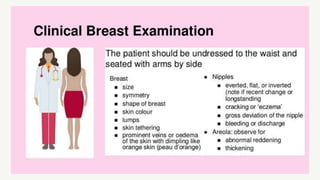
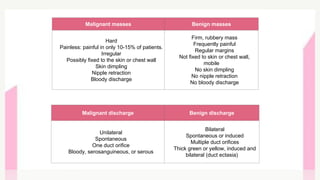
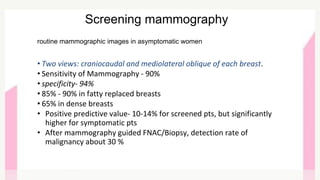
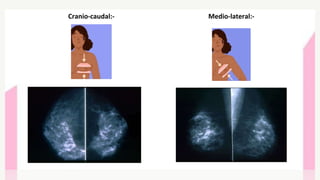
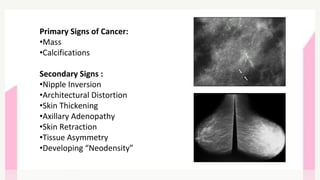
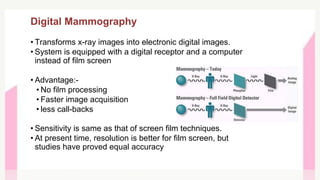
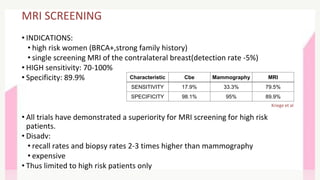
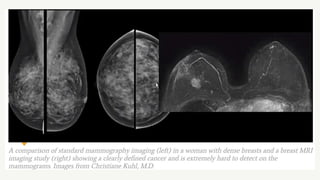
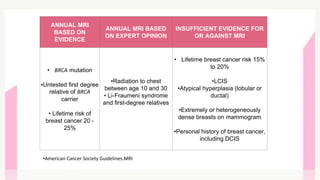
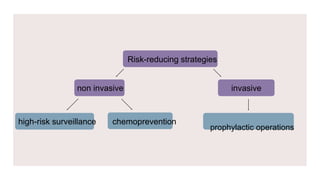
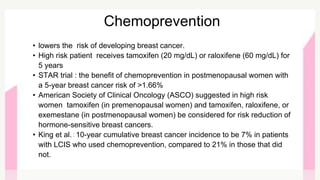
This document provides information on breast cancer screening and prevention. It discusses screening principles and guidelines for mammography, MRI, ultrasound and other screening techniques. It outlines high-risk factors for breast cancer and recommends annual screening starting at age 30-40 for high-risk individuals, including those with BRCA gene mutations or family history. Screening mammography every 1-2 years is recommended for average risk women starting at age 40. Chemoprevention with tamoxifen or raloxifene can lower breast cancer risk in high risk postmenopausal women. Genetic testing guidelines are also provided.