Thermodynamic processes involve a change in state of a system through heat or work interactions with the surroundings. Some common processes include:
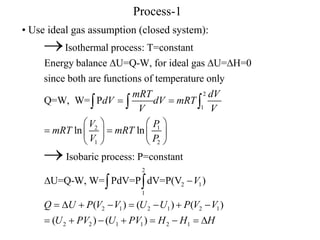
- Isothermal processes occur at constant temperature. For an ideal gas, heat and work are equal during these processes.
- Isobaric processes occur at constant pressure. For an ideal gas, work done is equal to the pressure-volume area.
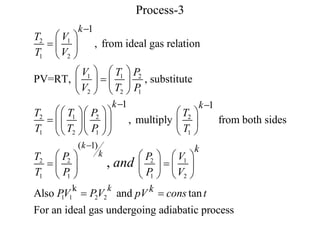
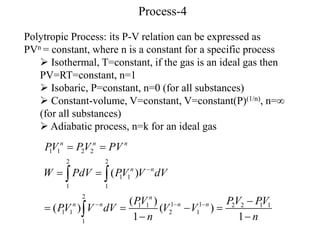
- Adiabatic processes involve no heat transfer. For an ideal gas undergoing adiabatic changes, the pressure-volume ratio remains constant.