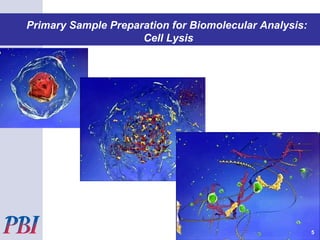
This presentation by Pressure BioSciences discusses their Pressure Cycling Technology platform for biological sample preparation. Some key points:
- PCT uses ultra-high pressure to break up cell walls and extract biomolecules, achieving better results than mechanical methods.
- The market for biological sample preparation is multi-billion dollars. PCT addresses the need for improved sample prep methods.
- Over 275 PCT systems have been installed at over 150 customer sites. There are over 100 publications highlighting PCT's advantages.
- Recent accomplishments include a co-marketing agreement with SCIEX, a leader in analytical technologies, and sales of their new Barocycler 2320EXTREME system.