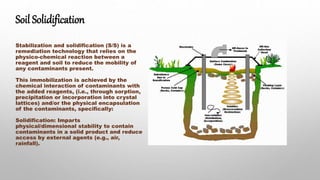
The presentation covers soil solidification, acidification, alkalization, and pollution, outlined as key topics in environmental geochemistry. Soil solidification is a remediation technology that immobilizes contaminants, while soil acidification and alkalization significantly impact agricultural productivity. Soil pollution poses health risks and threatens ecosystems due to contaminants from various human activities, with solutions aimed at reducing negative environmental impacts.