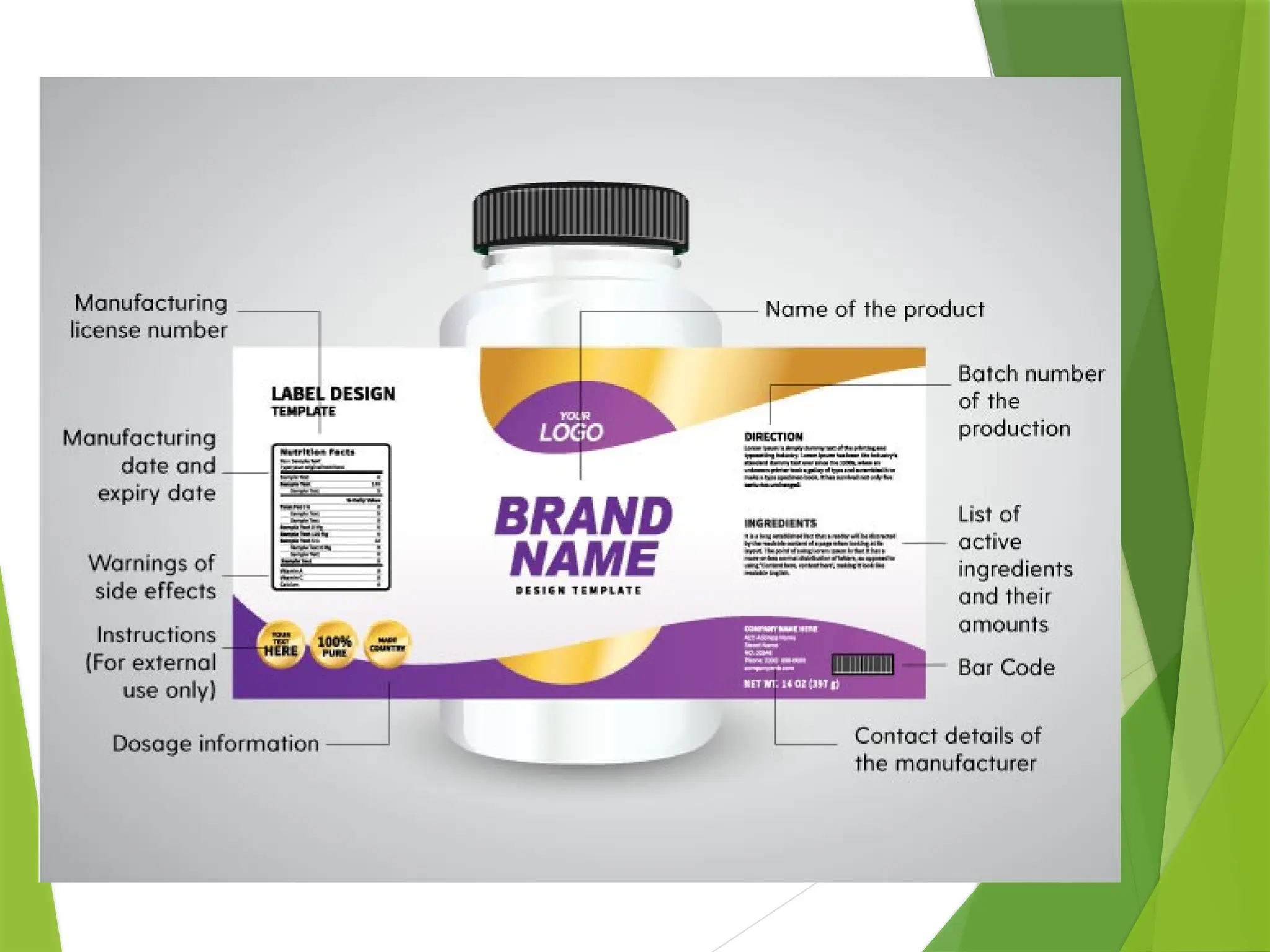
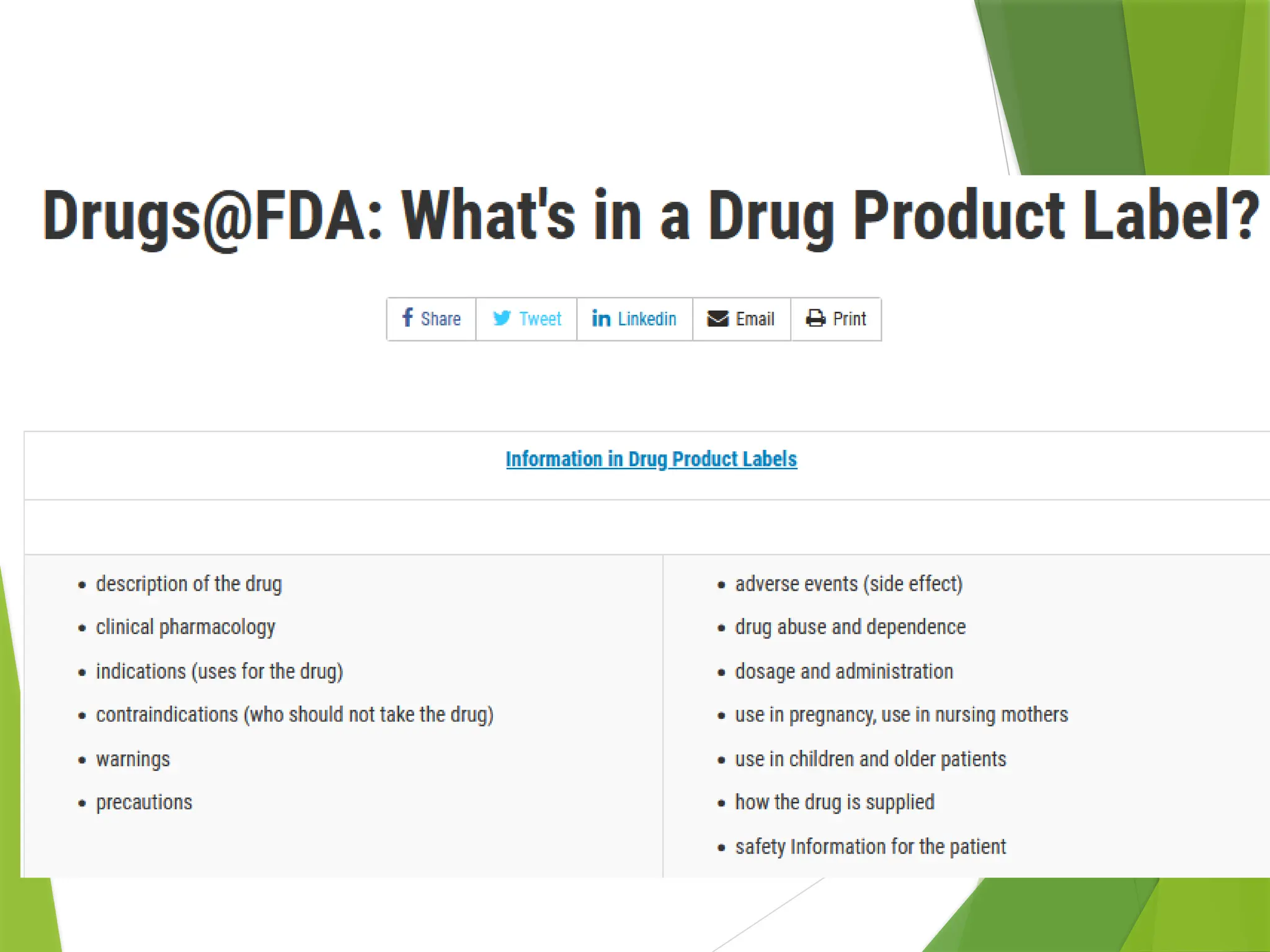
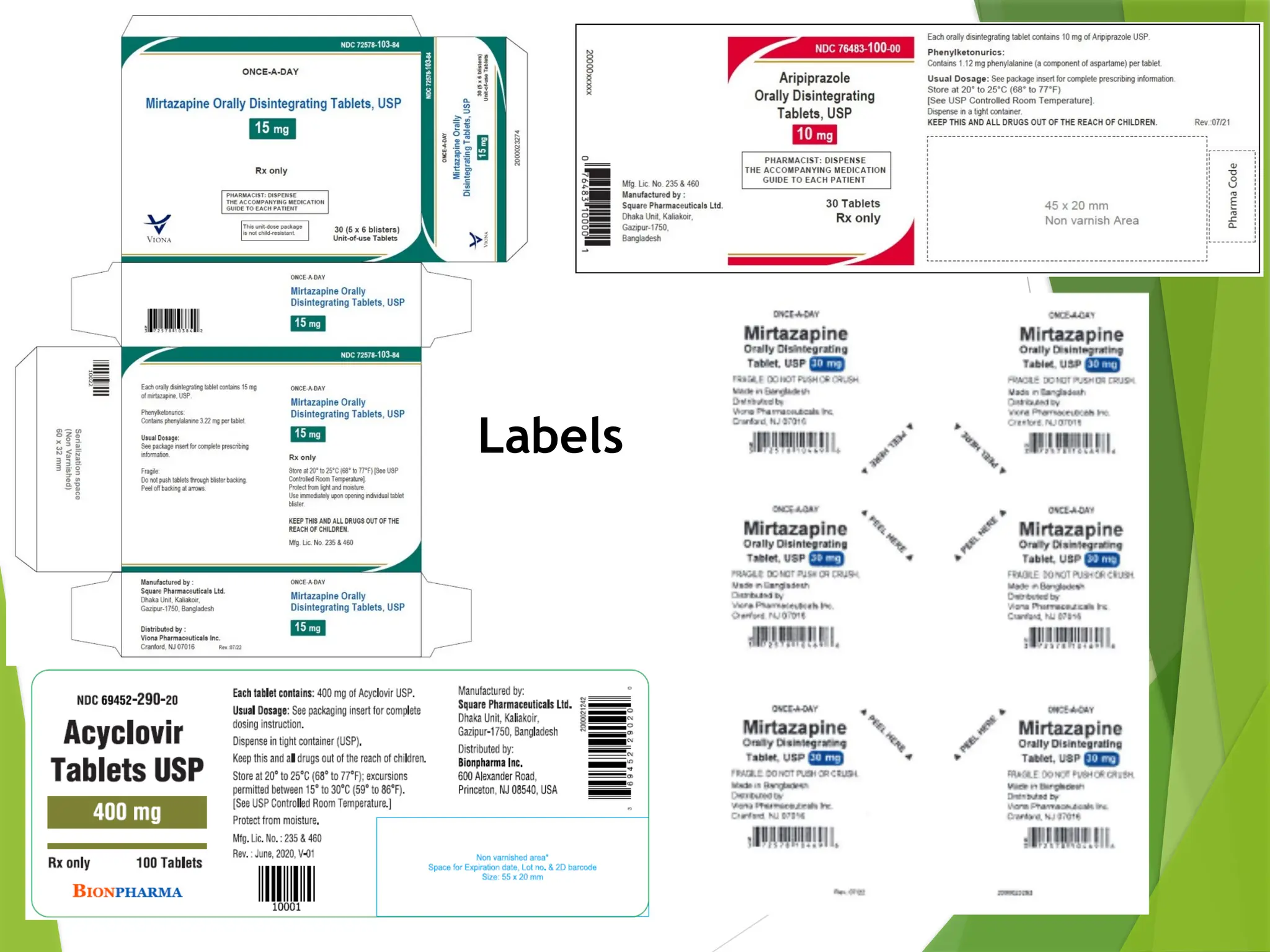
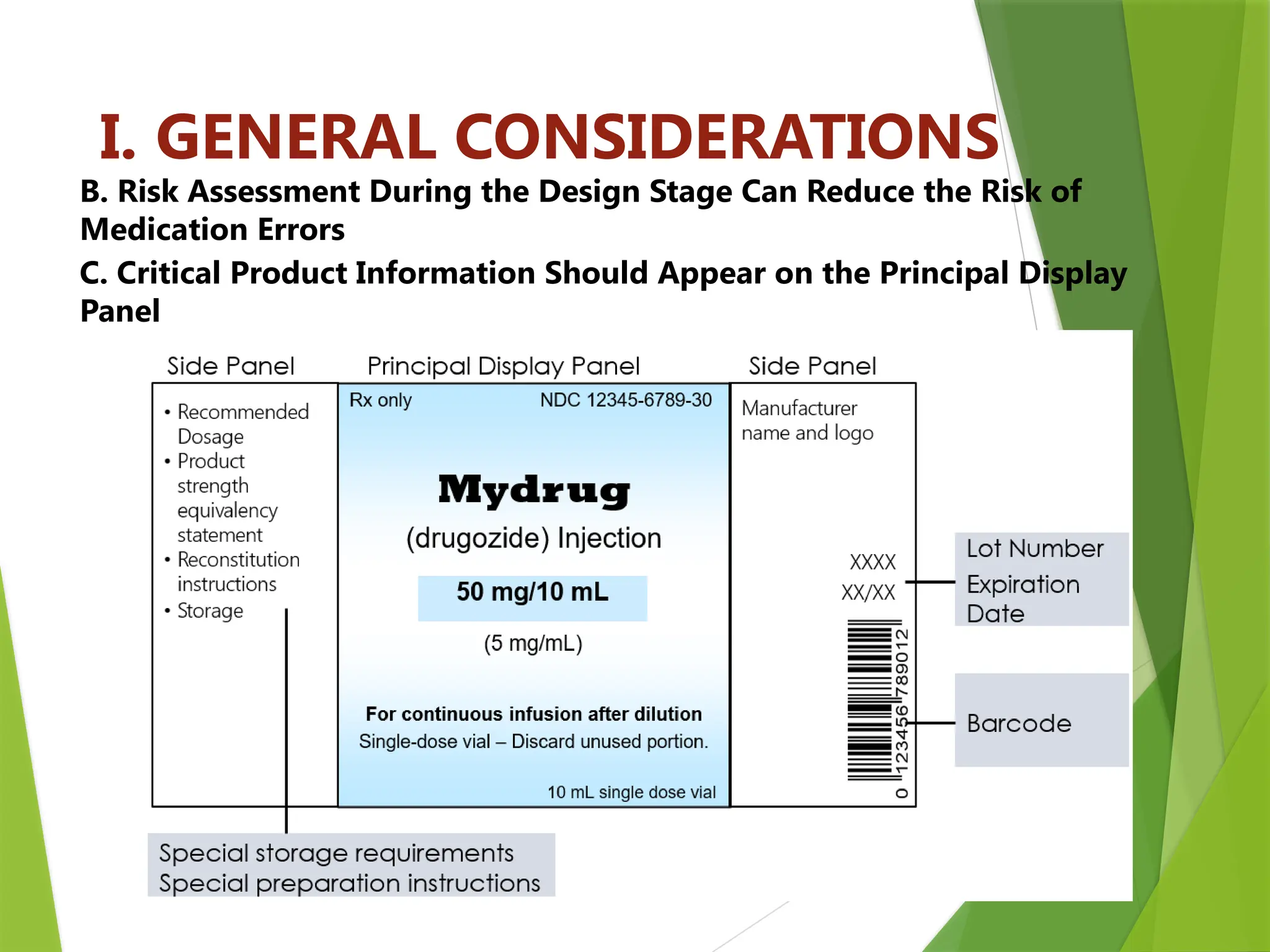
The document outlines principles and recommendations for the design and labeling of product containers and cartons to promote safe medication dispensing and administration. It emphasizes the importance of clear, legible labels to prevent medication errors and includes specific considerations regarding product strength, route of administration, and labeling for special packaging. Various design elements, like text size, color contrast, and avoiding confusing abbreviations, are critical for ensuring that critical safety information is effectively communicated.