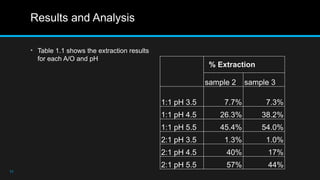
The document details a practical experiment on extracting cobalt from a pregnant leach solution using solvent extraction, particularly employing phosphinic acid (Cyanex 272) as an extractant. It outlines the methodology for preparing the cobalt sulfate solution, conducting the extraction, and analyzing the results which show that higher pH levels and specific aqueous-to-organic ratios enhance cobalt extraction efficiency. Recommendations for improving the extraction process are also provided, such as better mixing techniques and optimal timing for phase separation.