PPT on structure of atom.pptx
•Download as PPTX, PDF•
0 likes•2 views
atoms
Report
Share
Report
Share
Recommended
More Related Content
Similar to PPT on structure of atom.pptx
Similar to PPT on structure of atom.pptx (20)
Recently uploaded
Recently uploaded (20)
Fostering Friendships - Enhancing Social Bonds in the Classroom

Fostering Friendships - Enhancing Social Bonds in the Classroom
Food safety_Challenges food safety laboratories_.pdf

Food safety_Challenges food safety laboratories_.pdf
HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx

HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx
ICT Role in 21st Century Education & its Challenges.pptx

ICT Role in 21st Century Education & its Challenges.pptx
21st_Century_Skills_Framework_Final_Presentation_2.pptx

21st_Century_Skills_Framework_Final_Presentation_2.pptx
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...

Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...
Basic Civil Engineering first year Notes- Chapter 4 Building.pptx

Basic Civil Engineering first year Notes- Chapter 4 Building.pptx
Beyond_Borders_Understanding_Anime_and_Manga_Fandom_A_Comprehensive_Audience_...

Beyond_Borders_Understanding_Anime_and_Manga_Fandom_A_Comprehensive_Audience_...
PPT on structure of atom.pptx
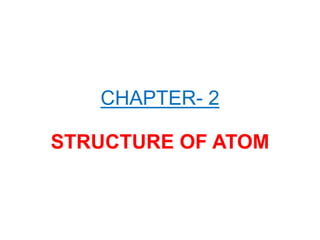
- 1. CHAPTER- 2 STRUCTURE OF ATOM
- 2. Matter Anything that has mass and occupies space. Matter is made up of tiny particles called atoms. Atom Atom is a smallest unit of matter. They can participate in a chemical reaction.
- 3. Dalton’s Atomic Theory In 1808 John Dalton proposed the theory Main postulates of the theory: 1.Matter is made up of small indivisible particles called atoms. 2. It is considered as a atom occurs in spherical shape or a indivisible particle of matter. 3. Atoms can neither be created nor be destroyed. 4. Atoms of different element have different properties. 5. All the atoms of a given element having same atomic masses but having different properties.
- 4. Discovery of Electron Sir J J Thomson was discovered electron by cathode ray tube experiment. Cathode ray tube is made up of glass containing two thin pieces of metal, called electrodes, sealed in it. The electrical discharge through the gases could be observed only at very low pressures and at very high voltages. The pressure of different gases could be adjusted by evacuation of the glass tube. When sufficiently high voltage is applied across the electrode, current starts flowing through a steam of particles moving in the tube from negative electrode to the positive electrode. These were called cathode rays or cathode ray particles.
- 5. Drawbacks of Dalton’s Atomic Theory 1. The indivisibility of atom was proved wrong because atom can be further divided into electron, proton and neutrons. 2. Elements having same atomic number but different atomic masses are called isotopes. 3. Elements having same atomic masses but having different atomic numbers are called isobars. 4.This theory fails to explain the existence of allotropes.
- 6. Properties of Cathode Rays 1. Cathode rays travel in a straight line. 2. Cathode rays are made up of negatively charged particles. 3. Cathode rays deflected towards magnet. 4. Cathode rays showing heating effect. 5. Cathode rays ionize the gases through which they travel.
- 7. Multiple Choice questions: 1. Who discovered electron ? (a) JJ Thomson (b) James Chadwick (c) Neil Bohr 2. To provide low pressure in the discharge tube we attach__ (a)heat (b)vacuum pump (c) electrodes 3. To discover electrons gas discharge tube is fitted with __ (a) two electrodes (b) three electrodes (c) four electrodes 4. Neutrally charged particles are protons. (a) True (b) False 5. Elements having same atomic number but different atomic mass are called___ (a) Isotopes (b) Isobars
- 8. THANK YOU