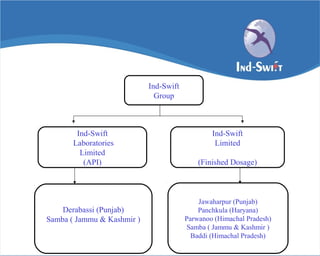
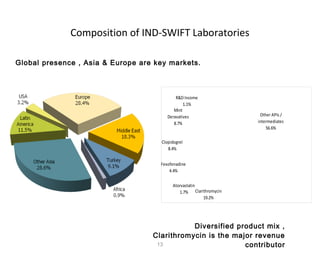
The document summarizes Ind-Swift Laboratories Limited, a pharmaceutical group based in India. Some key points:
- It was founded in 1986 and has grown to a consolidated turnover of around Rs. 2200 Crores with businesses in APIs, chemicals, infrastructure, education, and media.
- It is a leading research-driven pharmaceutical group operating in over 56 countries and catering to regulators like USFDA, MHRA, and others.
- The group has manufacturing facilities in India and wholly owned subsidiaries in the US, UK, Iran, Dubai, and Singapore.
- It manufactures over 40 API products across 16 therapeutic categories and has international approvals from regulators in the US,