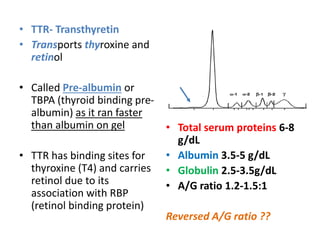
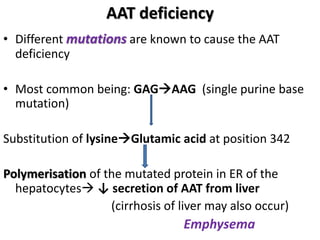
The document provides an overview of plasma proteins, including their classification, functions, and clinical significance. It discusses the synthesis of plasma proteins, their roles in inflammation, transport functions, and therapeutic uses, as well as specific proteins such as albumin, ceruloplasmin, and α1-antitrypsin. Additionally, it explains the implications of protein levels in various diseases and conditions, including the importance of albumin in maintaining osmotic pressure and the role of acute phase proteins in response to inflammation.