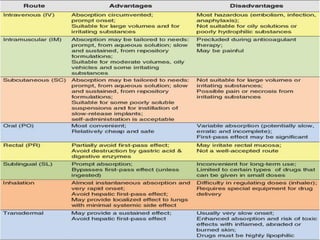
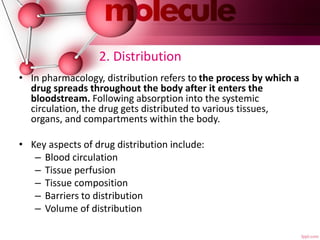
The document provides an overview of pharmacokinetics, detailing the processes of absorption, distribution, metabolism, and excretion of drugs. It emphasizes the importance of understanding how drugs are processed in the body to optimize therapy, minimize side effects, and predict drug interactions. Factors affecting each of these processes, such as route of administration, drug properties, and individual patient variables, are also discussed in depth.