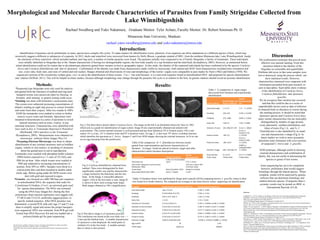
Morphological and molecular analysis was used to identify parasites collected from Lake Winnibigoshish in Minnesota. Parasites were stained and examined under microscopy to measure morphological characteristics, which supported identification as Cotylurus brevis, Cotylurus flabelliformis, and Apatemon gracillis based on comparisons to previous studies. Genetic sequencing of the COX1 gene was initiated but not completed. Results from staining were consistent with identification of the three species based on features such as testis shape, ovary placement, and body ratios being within reported ranges. Molecular analysis may further support identifications but has not been finished.