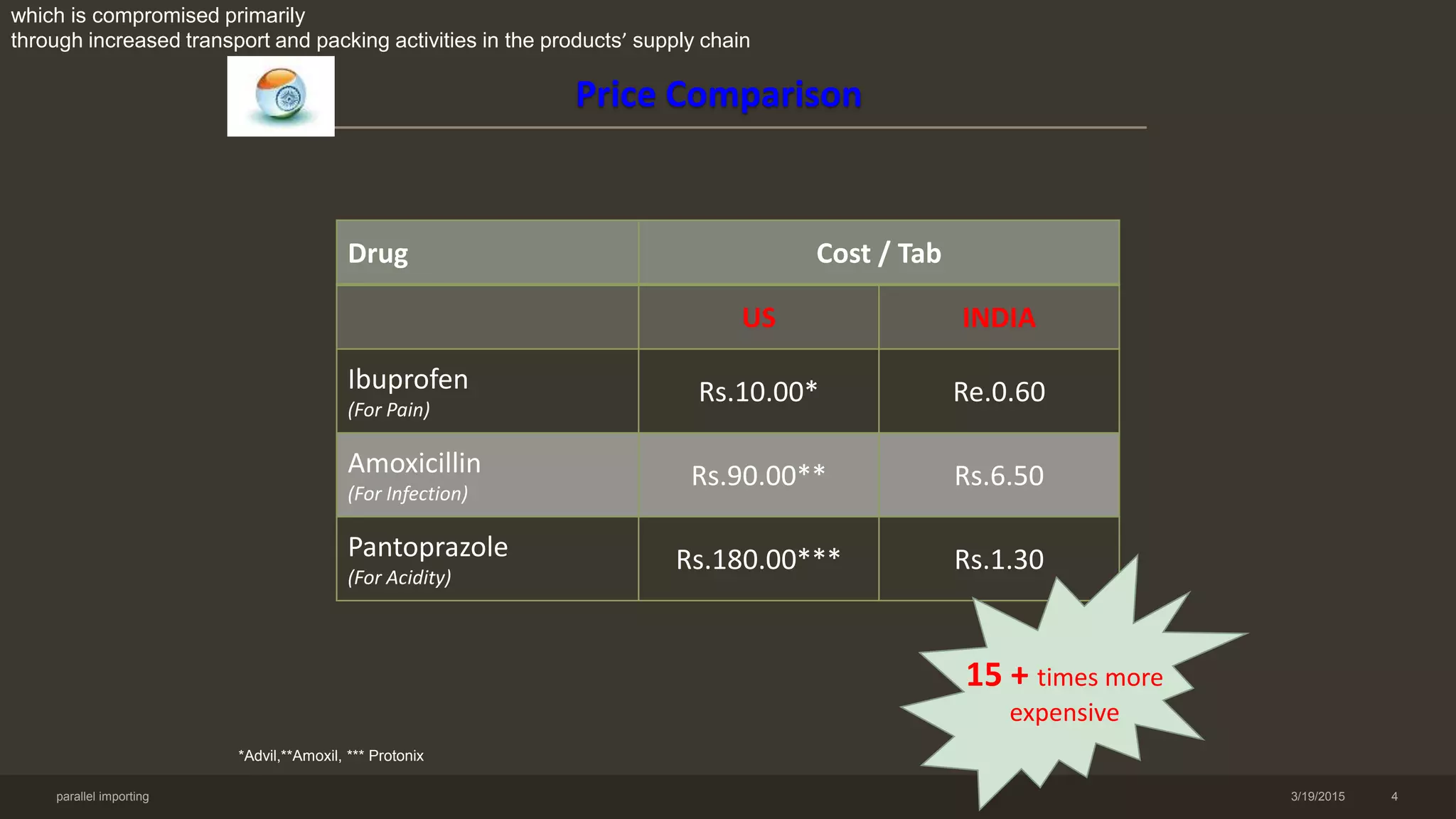
This document discusses parallel imports of pharmaceuticals. It begins by defining parallel imports as the importation of non-counterfeit products without the authorization of the patent holder, often occurring when prices vary between markets. It then examines reasons for price differentiations internationally and provides examples of large price differences for common drugs between the US and India. The document outlines arguments for and against parallel imports, different countries' legal approaches, and India's stance, concluding that parallel imports have limited practical utility for India.