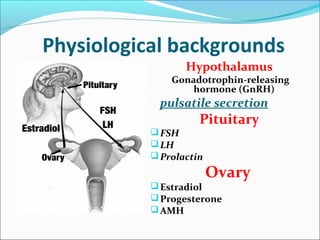
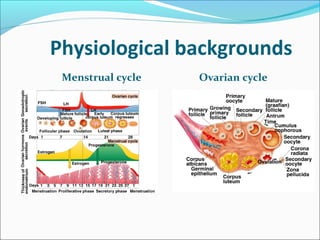
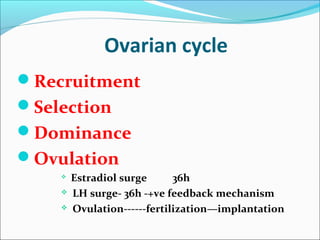
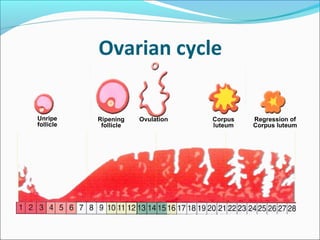
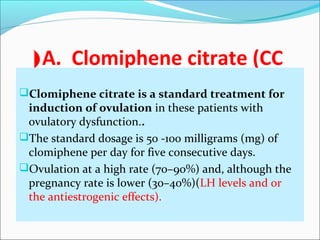
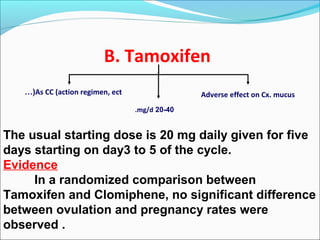
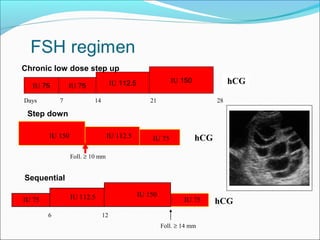
The document discusses various causes and treatments for infertility related to ovulation disorders. It defines types of anovulation according to the WHO classification and describes physiological processes related to the hypothalamic-pituitary-ovarian axis and menstrual cycle. Non-pharmacological treatments like weight loss and lifestyle changes are recommended as first-line options. Pharmacological treatments discussed include clomiphene citrate, tamoxifen, metformin, gonadotropins, and protocols for ovulation induction.