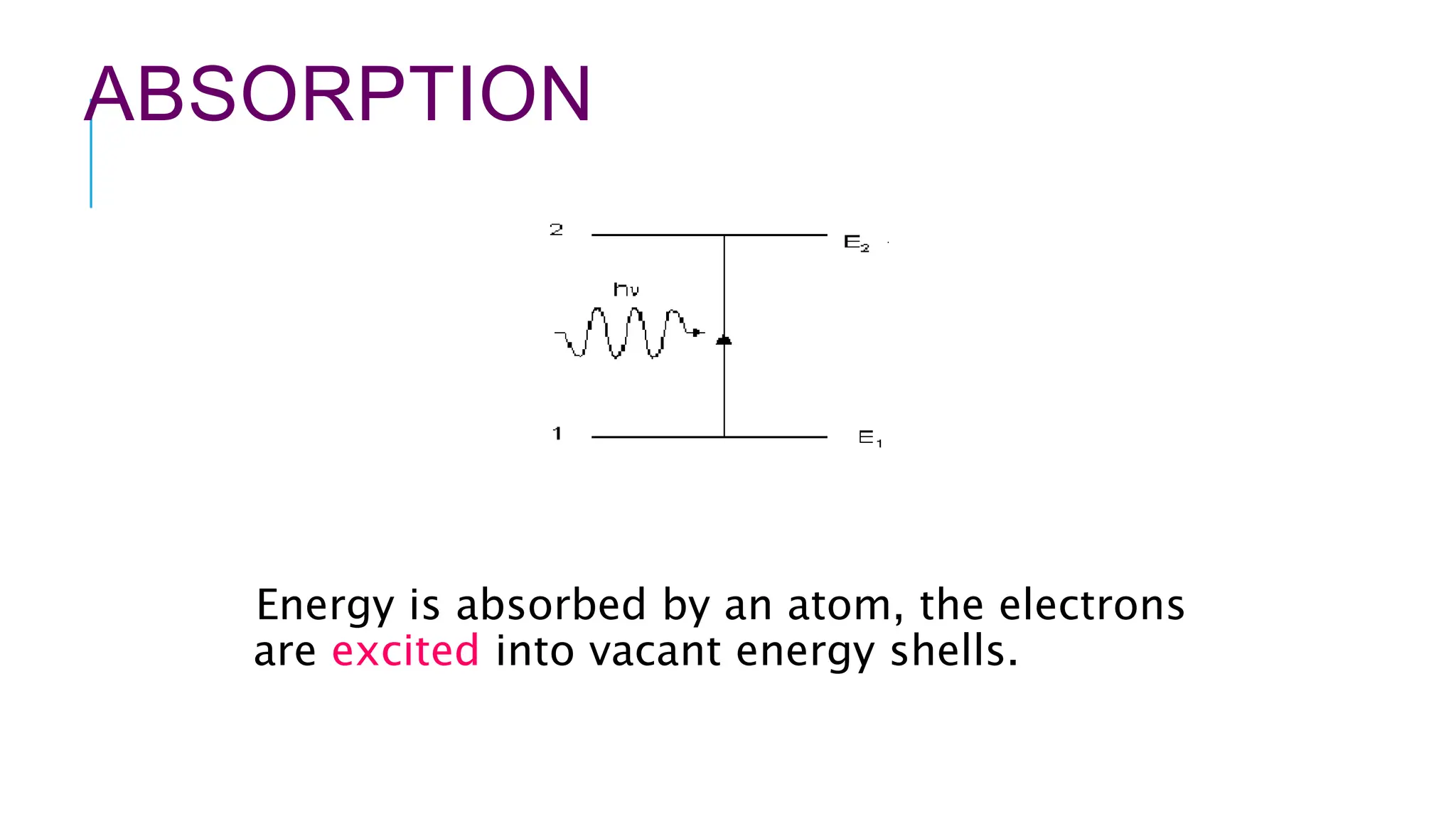
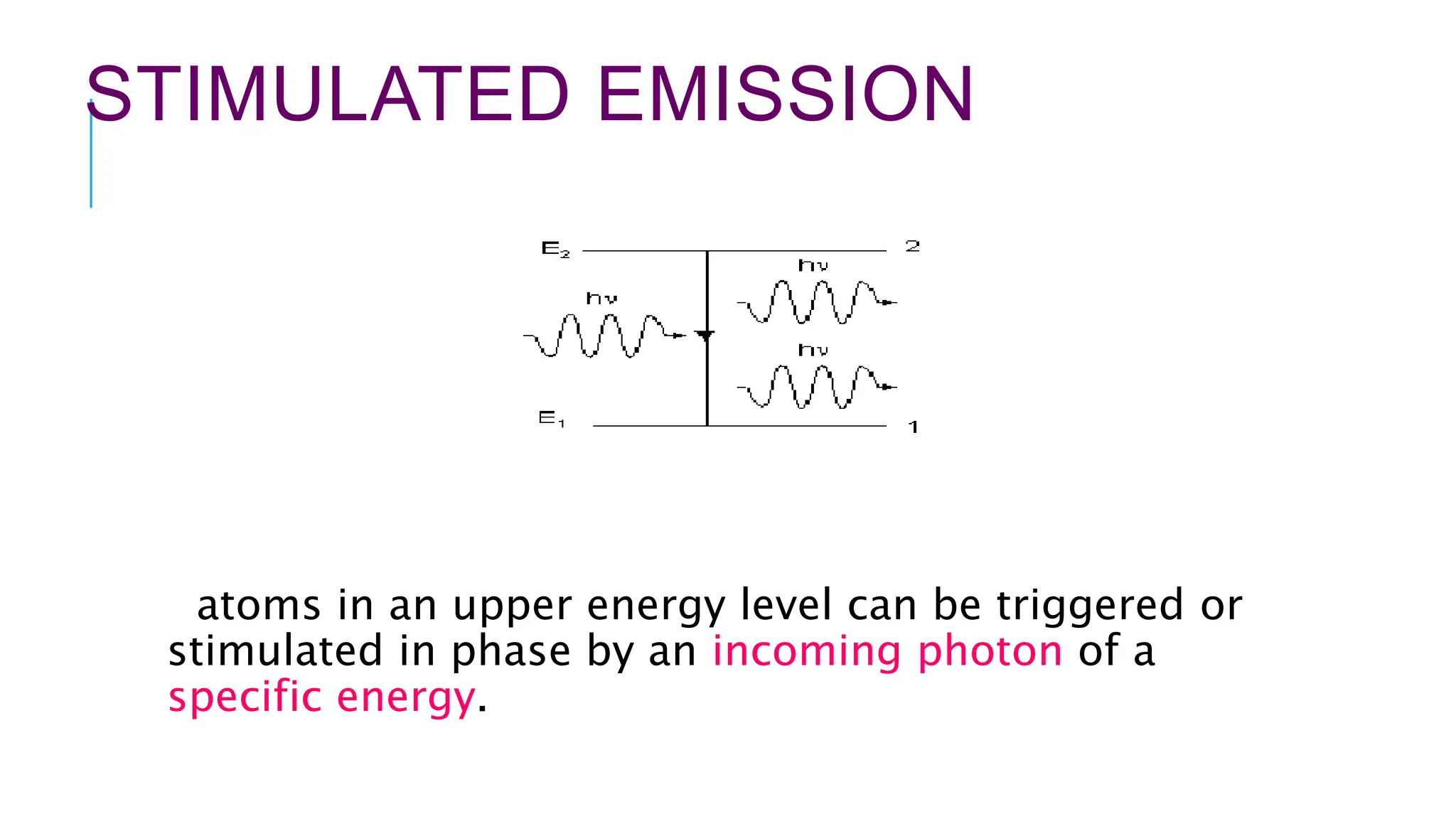
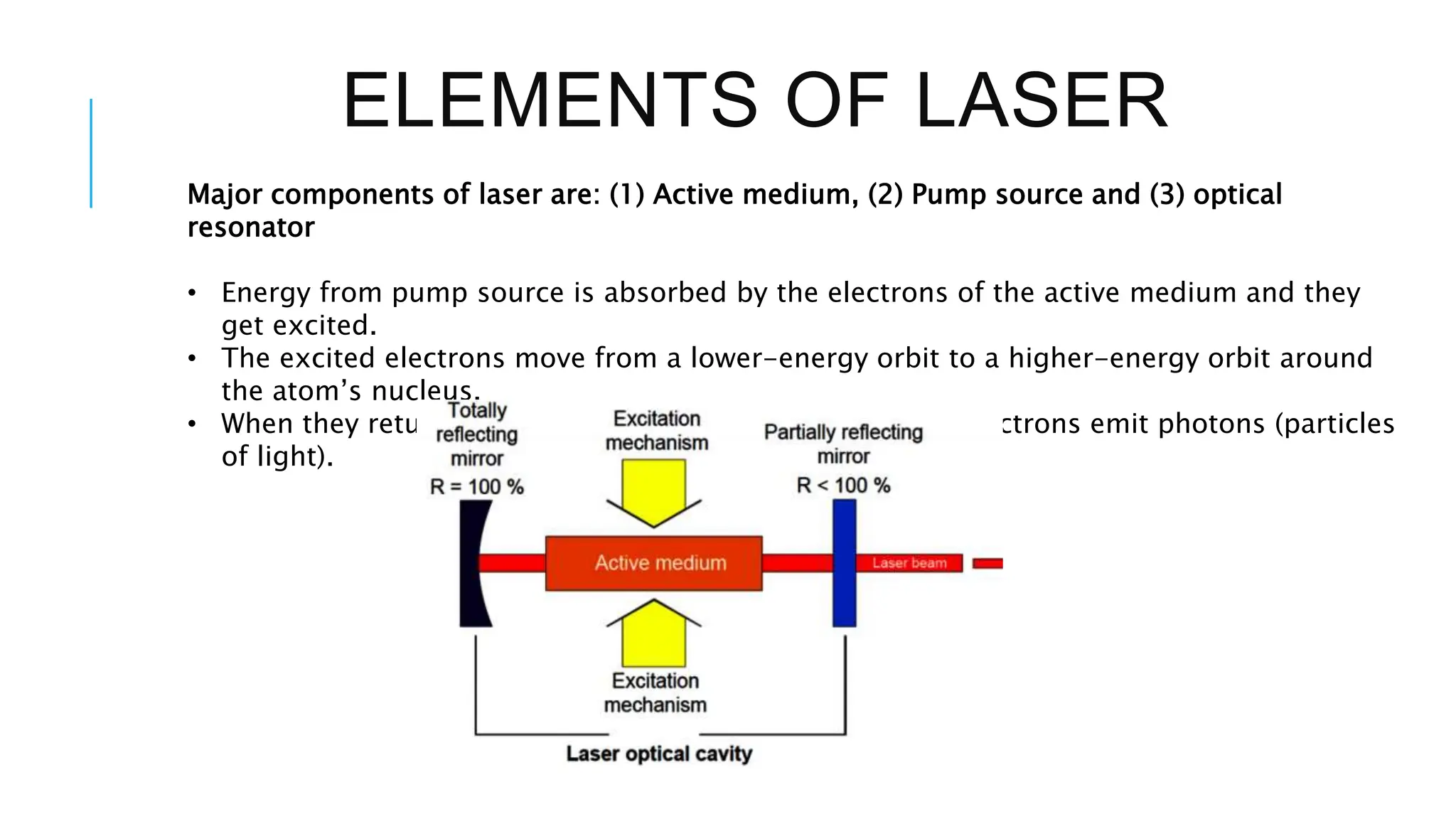
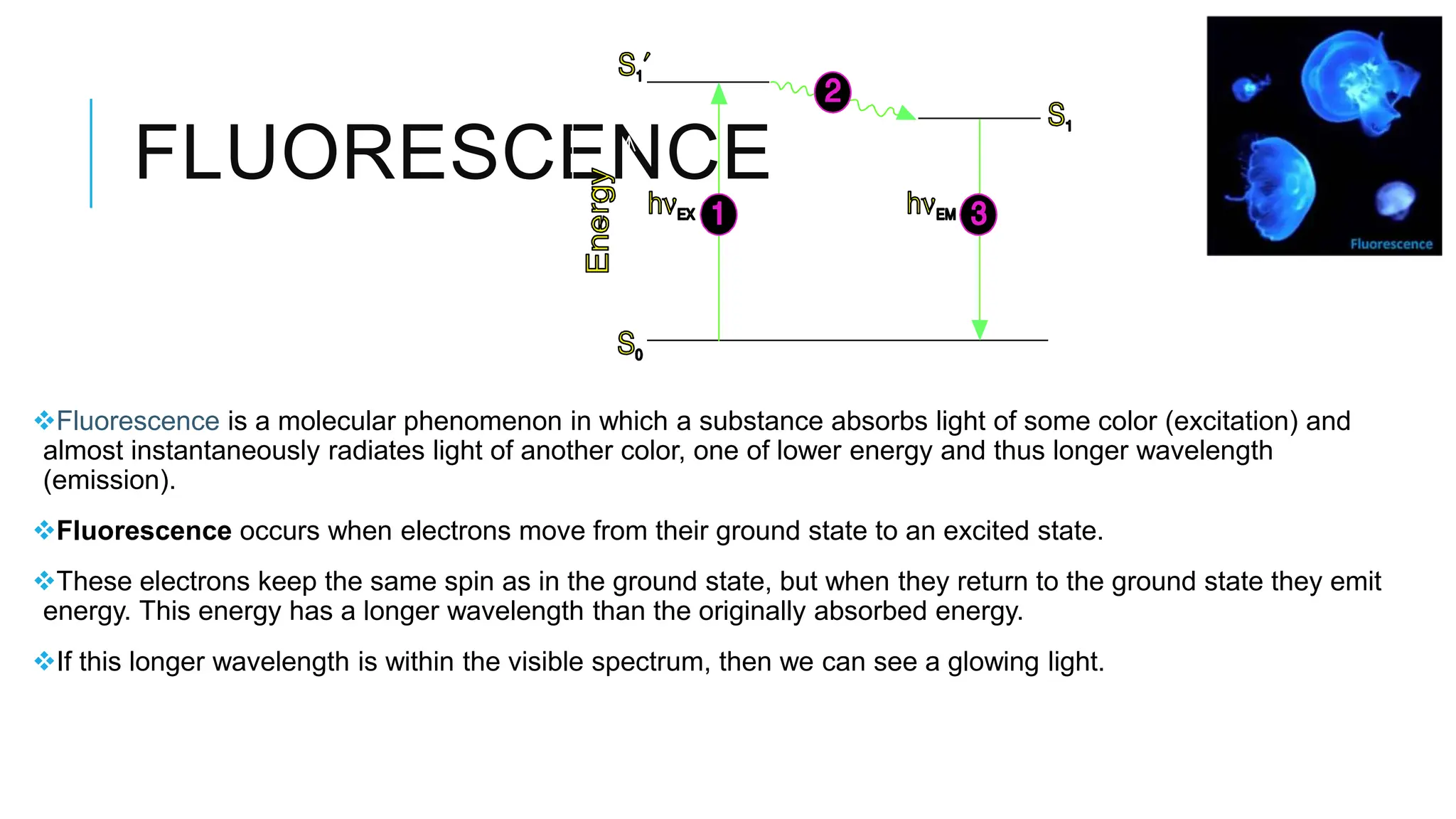
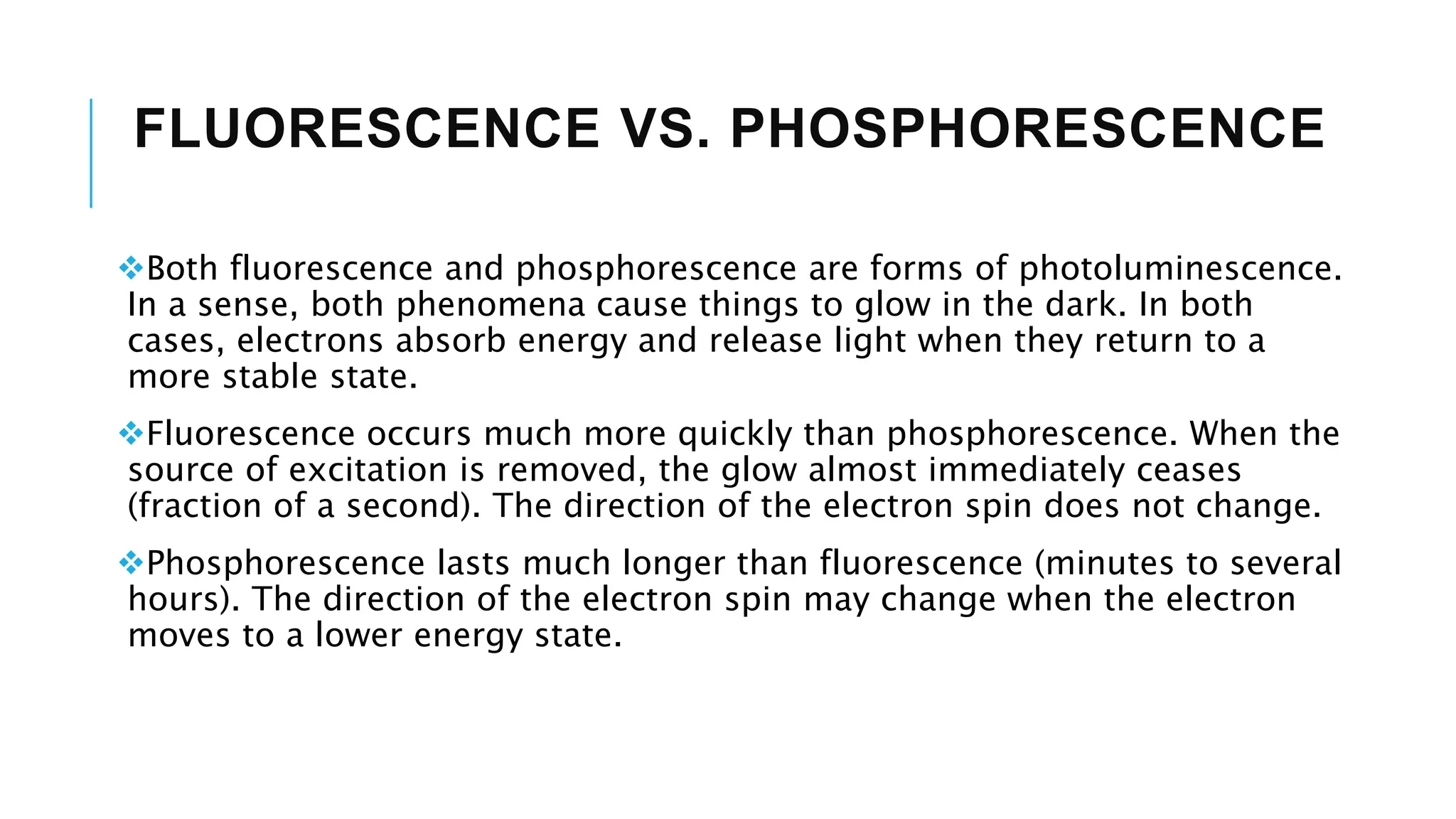
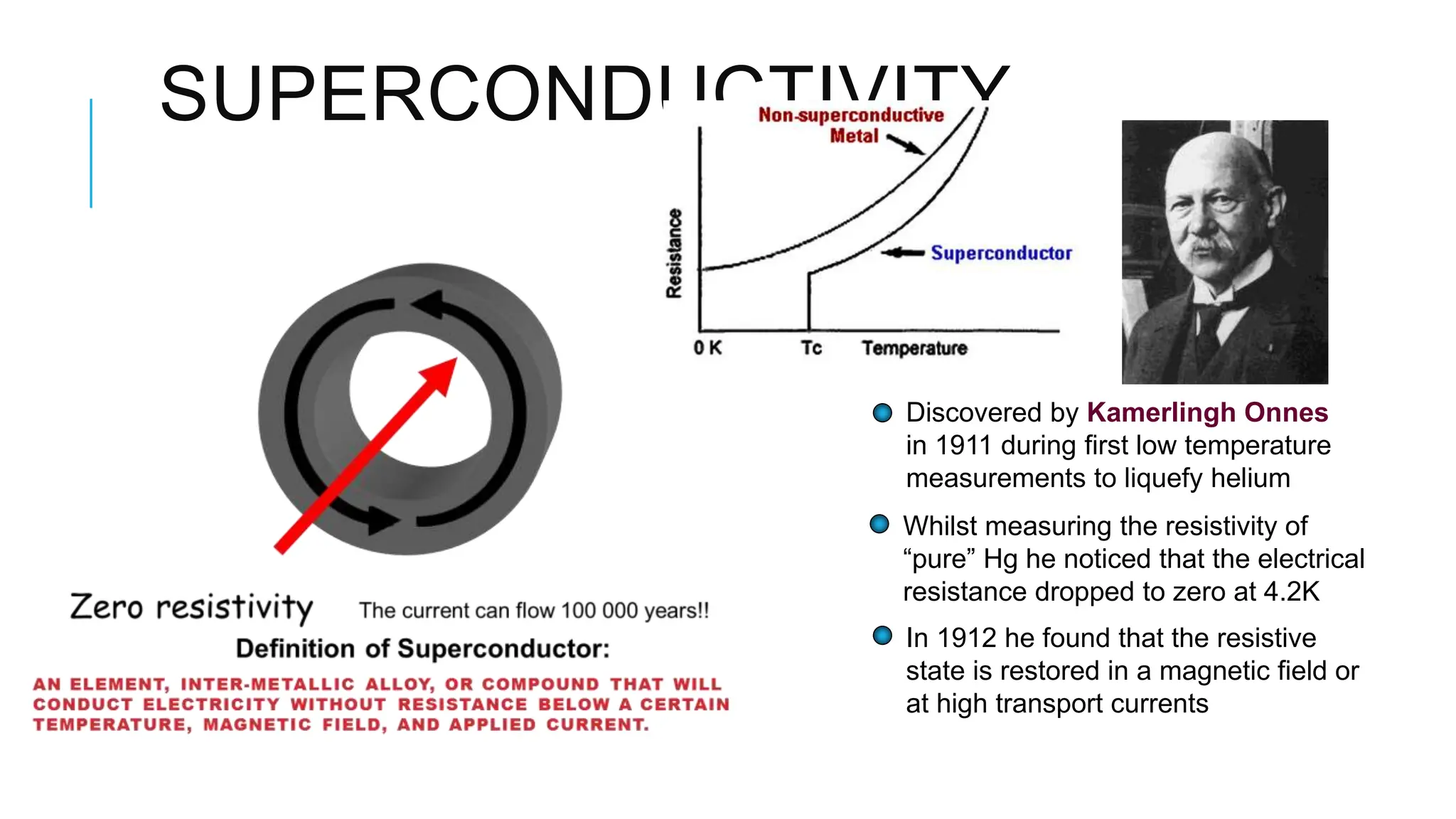
The document explains lasers, which produce coherent light through stimulated emission, emphasizing their properties of being monochromatic, highly directional, and coherent. It covers basic concepts such as absorption, spontaneous and stimulated emission, and the importance of population inversion, as well as the differences between fluorescence and phosphorescence in light emission processes. Additionally, it discusses the electromagnetic spectrum and applications of various types of electromagnetic waves, including radar and microwave technology.