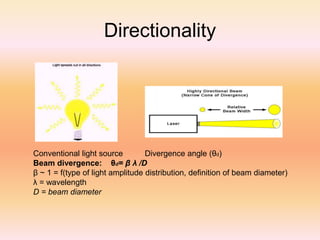
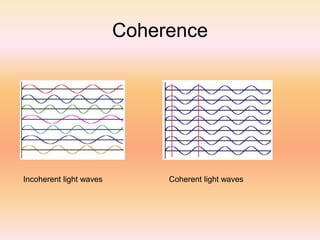
The document discusses lasers and their properties. It begins with the history of lasers, invented in 1958, and describes what a laser is - a device that produces coherent light through stimulated emission of radiation. The key properties of laser light are described as monochromatic, highly directional, and coherent. Ordinary light lacks these properties. The document then covers the basic concepts of how lasers work, including absorption, spontaneous emission, stimulated emission, population inversion, and pumping to achieve inversion. It describes various laser components and modes. In summary, the document provides an overview of the history and functioning of lasers.




















![The probability of spontaneous emission A2-1 /the probability of
stimulated emission B2-1r(n :
1. Visible photons, energy: 1.6eV – 3.1eV.
2. kT at 300K ~ 0.025eV.
3. stimulated emission dominates solely when hn /kT <<1!
(for microwaves: hn <0.0015eV)
The frequency of emission acts to the absorption:
if hn /kT <<1.
1
)
/
exp(
)
(
1
2
1
2
kT
h
B
A
n
n
r
1
2
1
2
1
2
1
2
2
1
1
1
2
2
1
2
2 ]
)
(
1
[
)
(
)
(
n
n
n
n
B
A
B
n
B
n
A
n
x
n
r
n
r
n
r
x~ n2/n1](https://image.slidesharecdn.com/laser-ppt-231003223639-26ccd339/85/laser-ppt-ppt-23-320.jpg)

