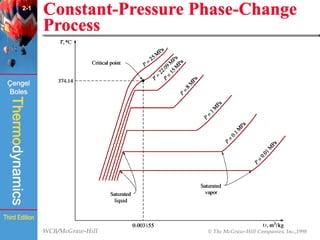
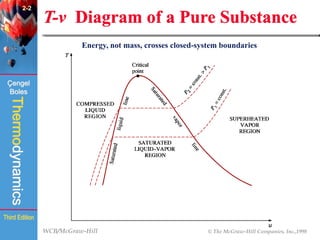
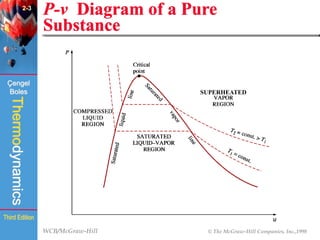
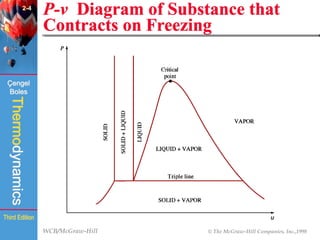
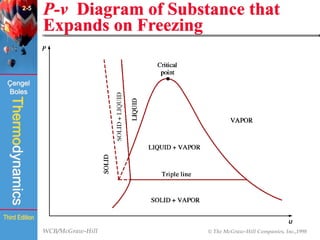
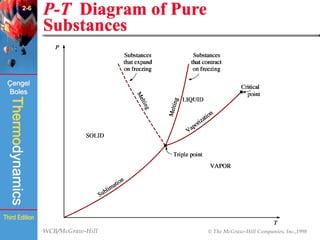
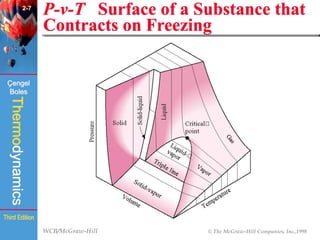
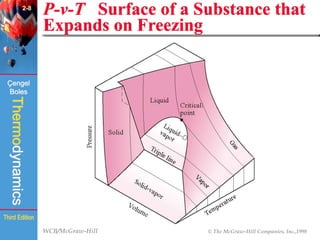
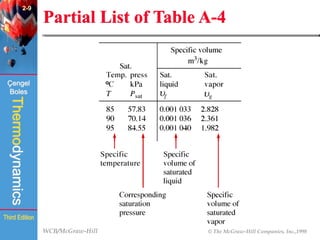
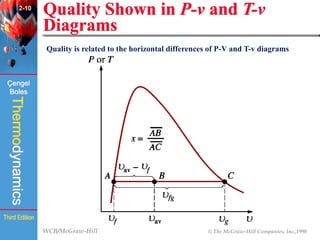
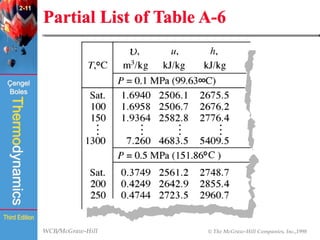
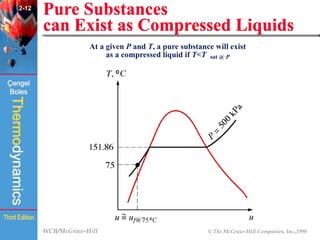
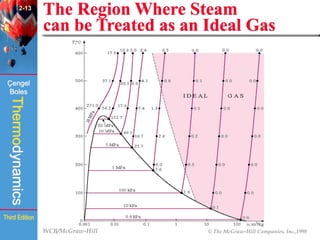
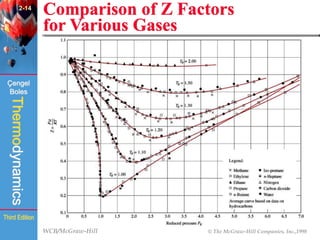
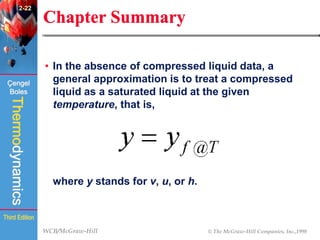
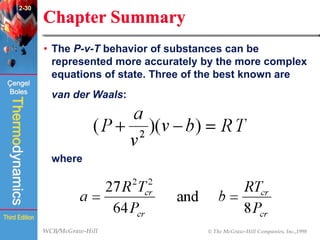
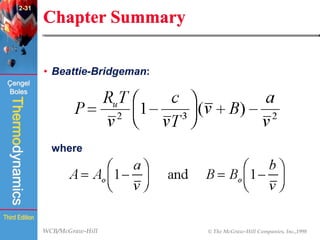
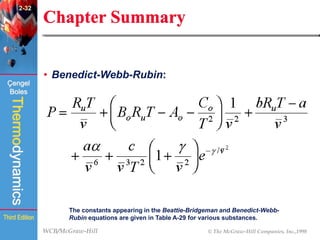
This document summarizes Chapter 2 of a thermodynamics textbook. It discusses properties of pure substances, including their different phases and how they change phases. It describes phase change processes and diagrams used to represent these processes. The summary also discusses equations of state that can be used to model the behavior of substances, like the ideal gas law and more complex equations that account for real gas behavior better. Key concepts covered include saturation temperature/pressure, quality, and using reduced properties and compressibility factors.