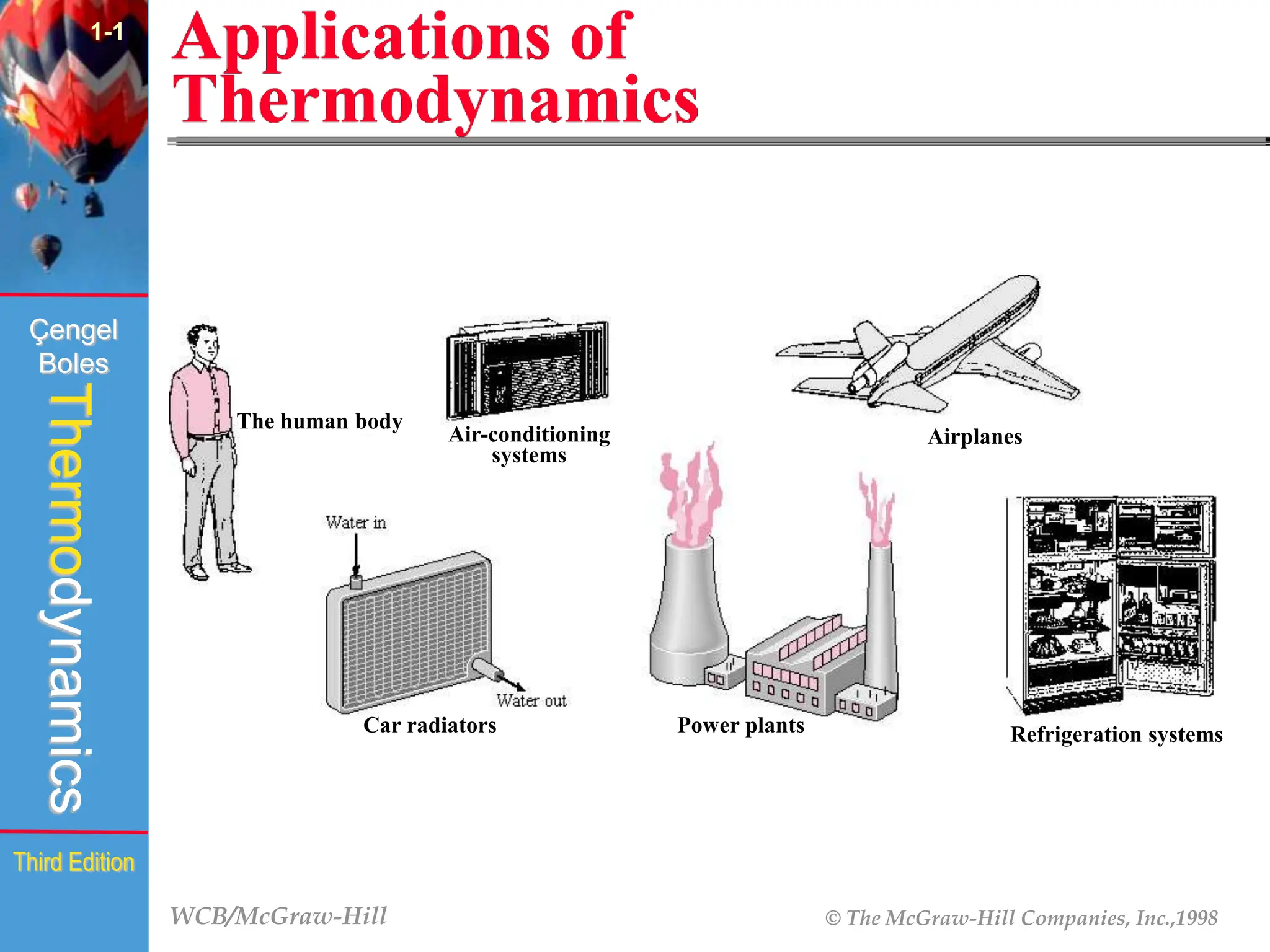
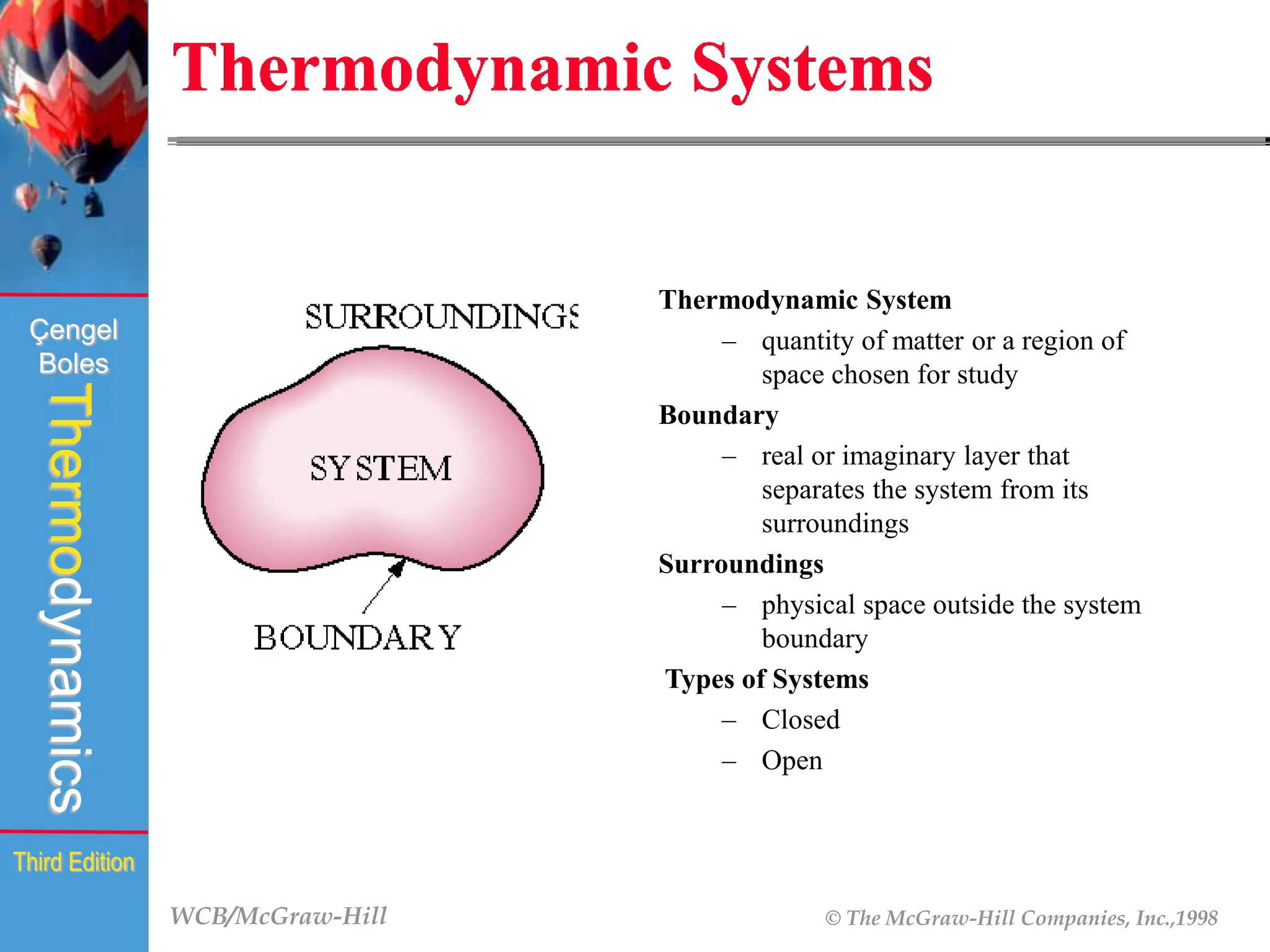
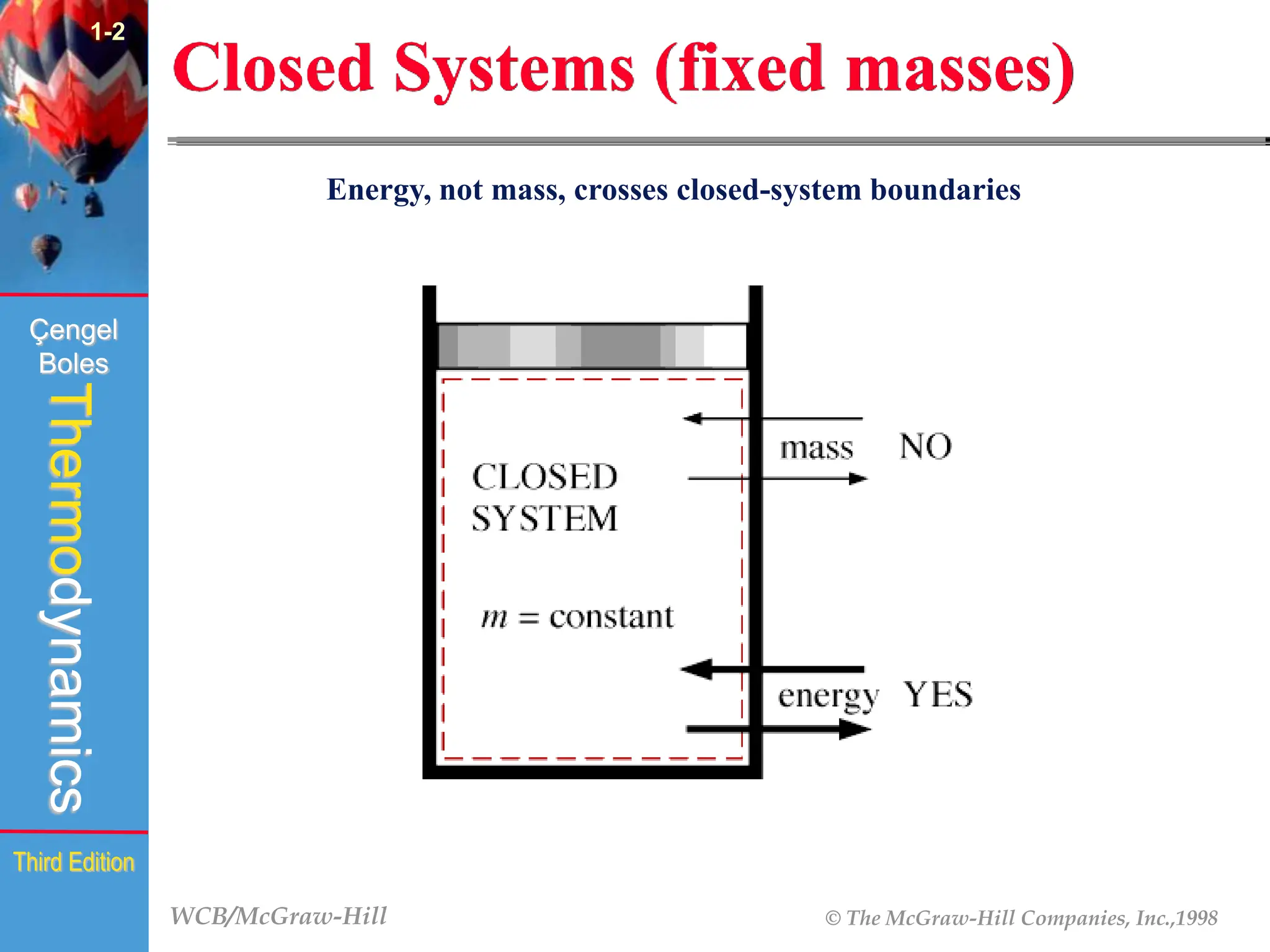
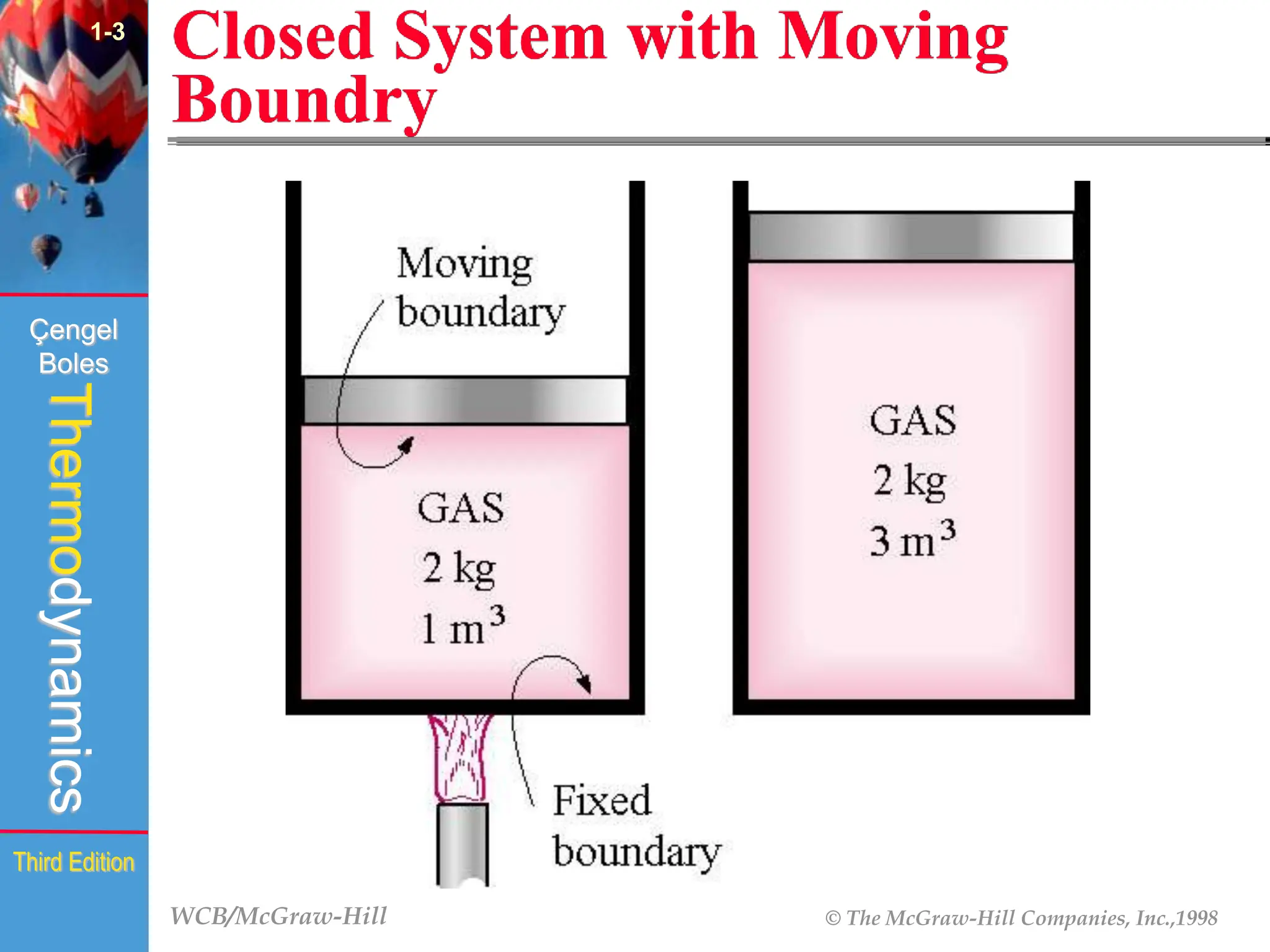
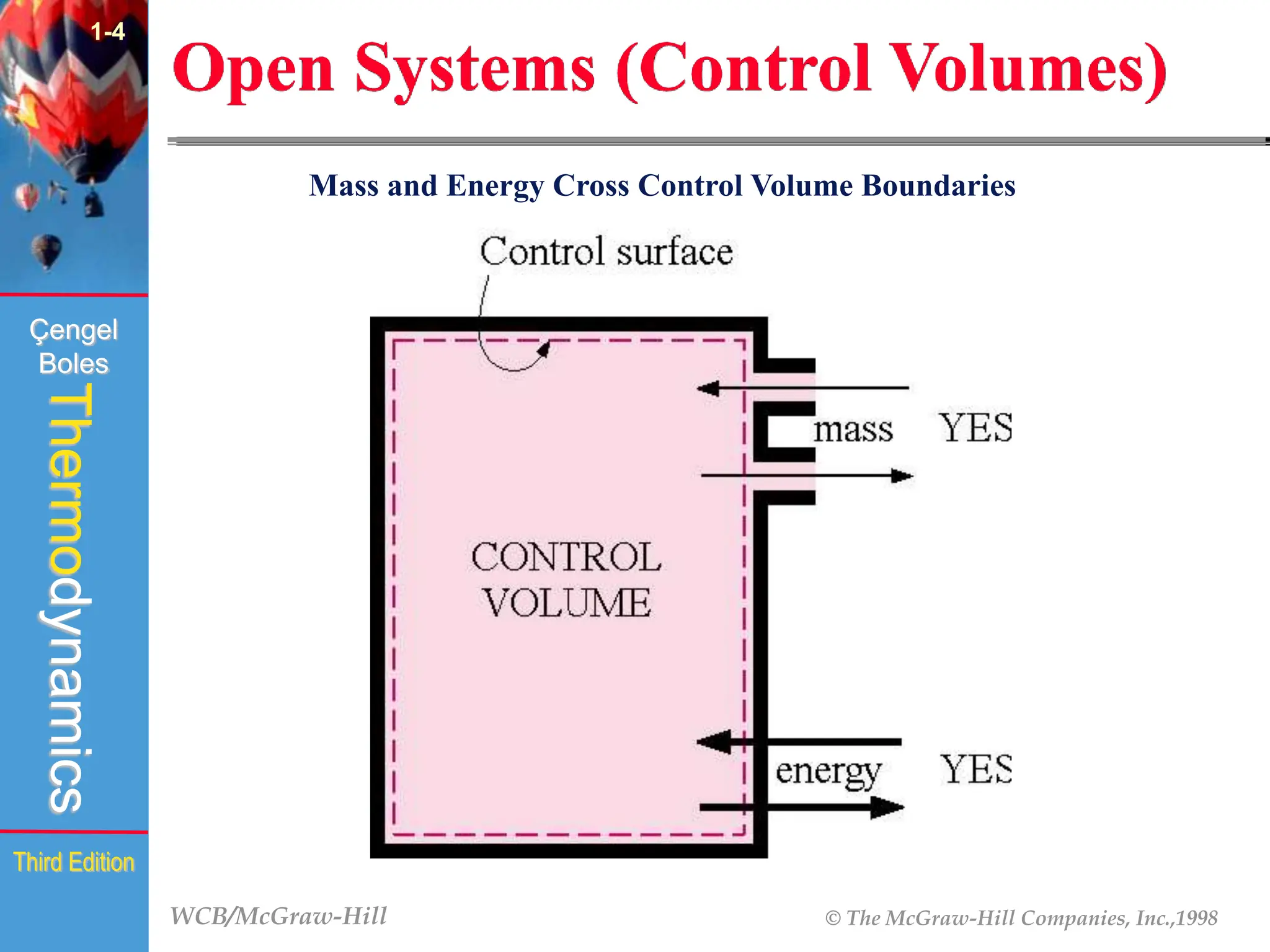
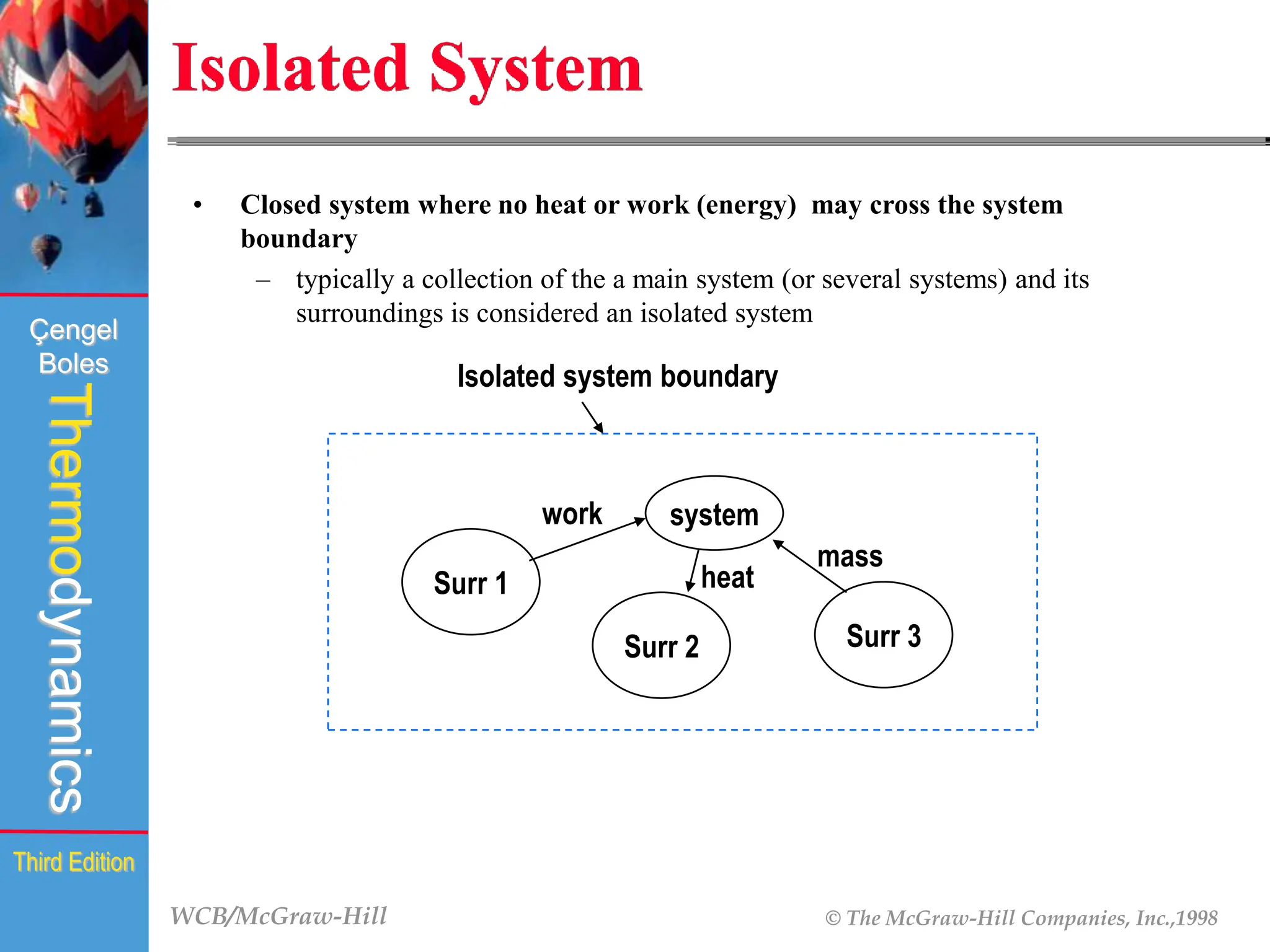
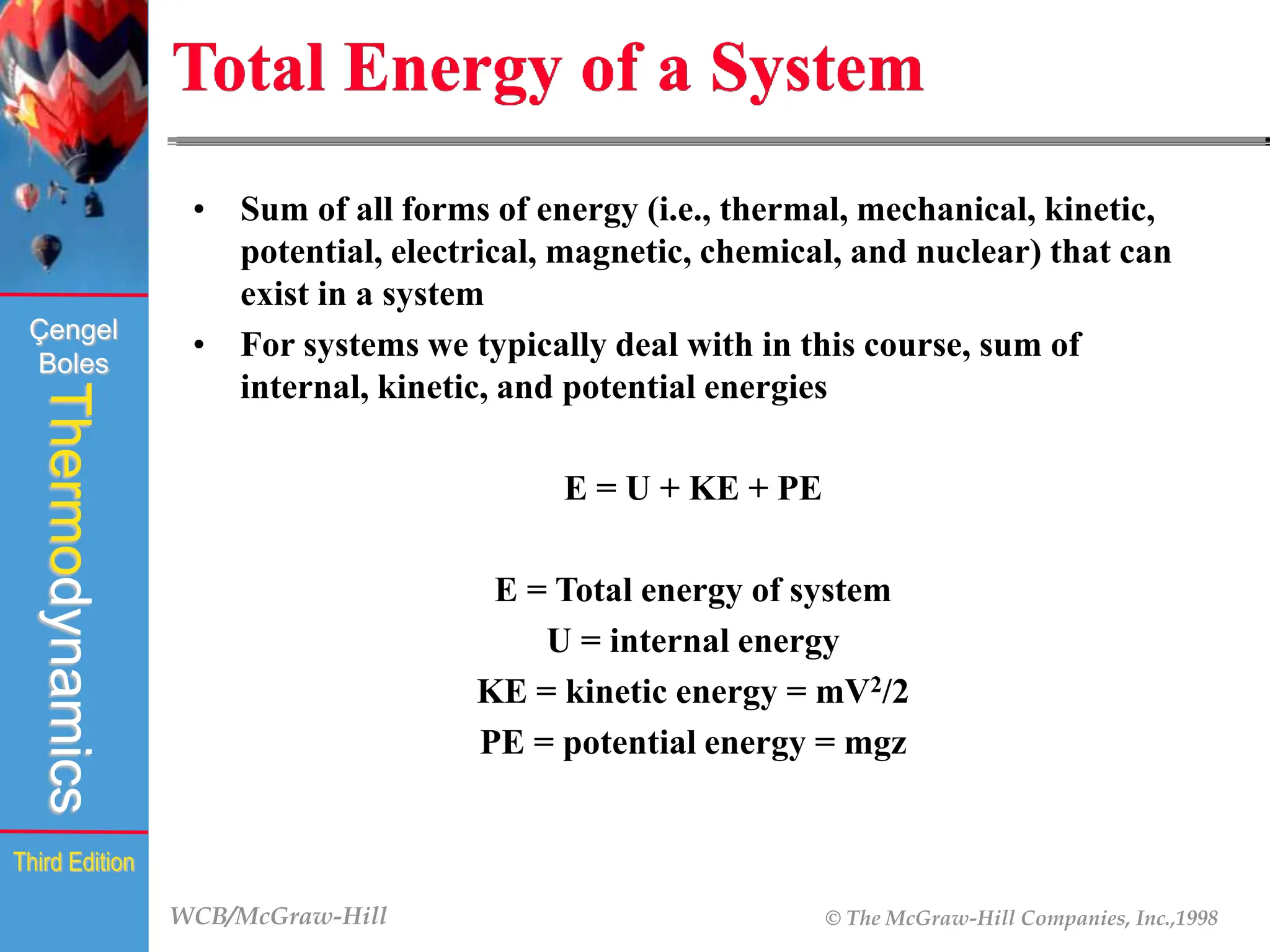
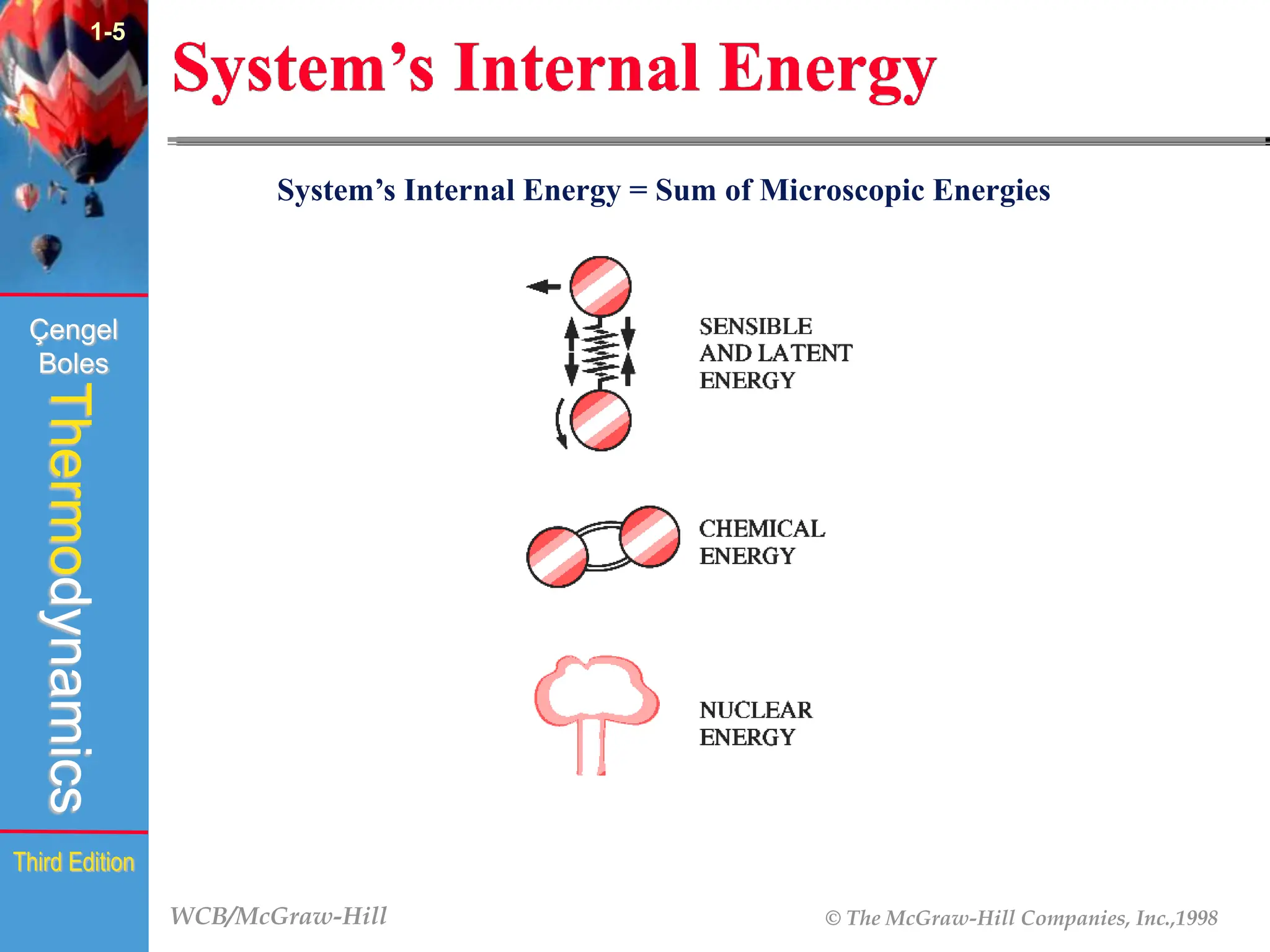
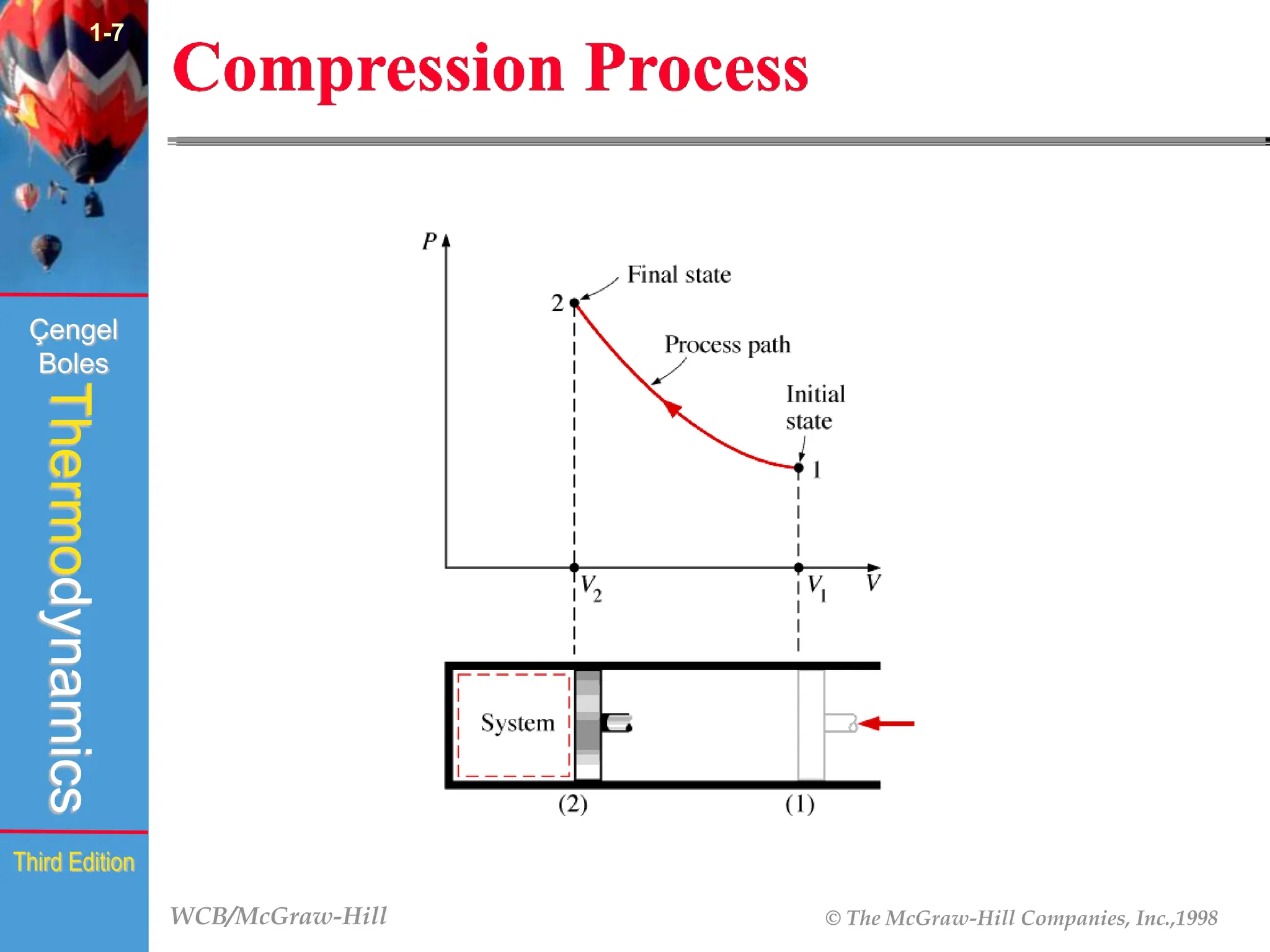
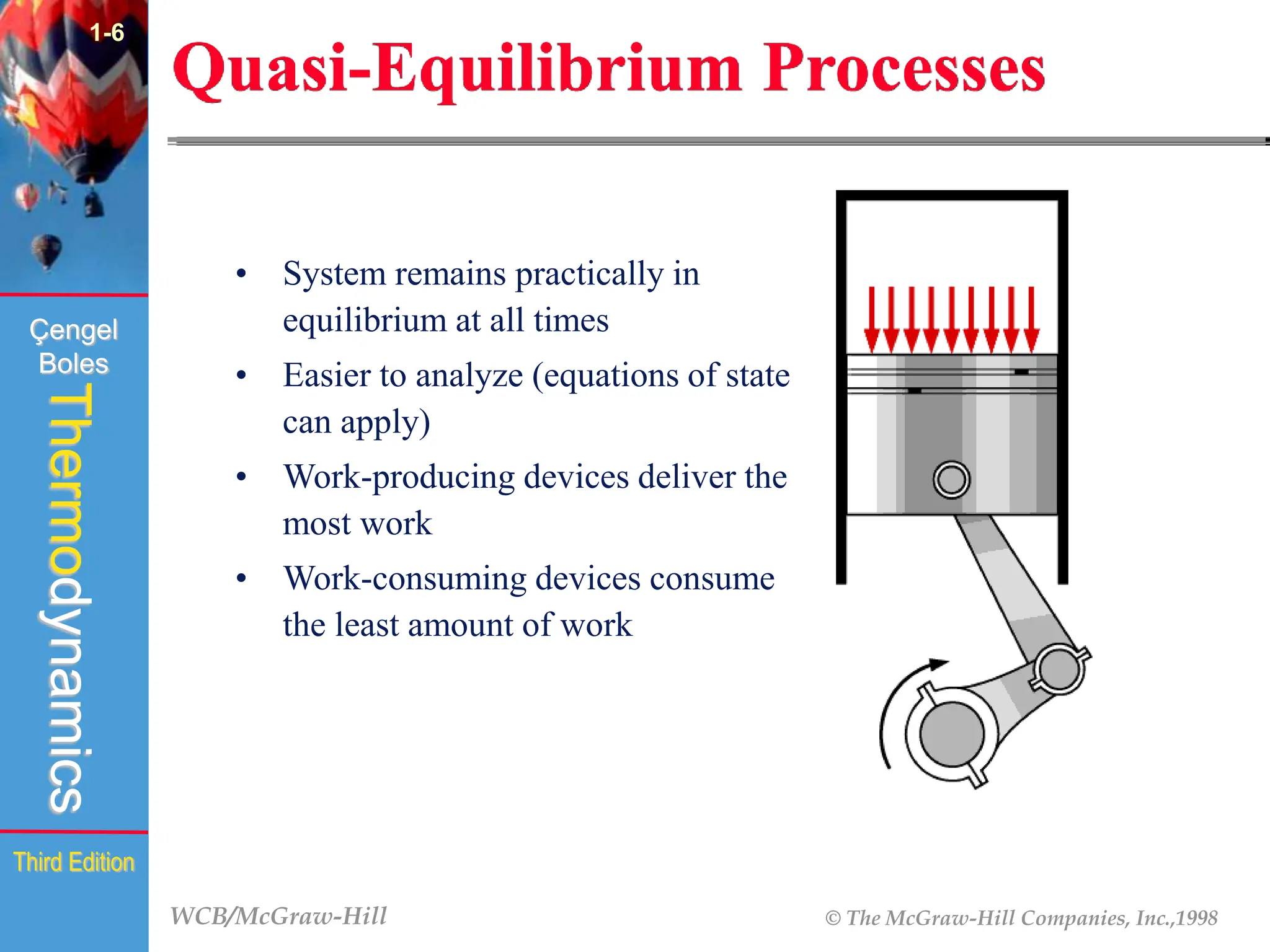
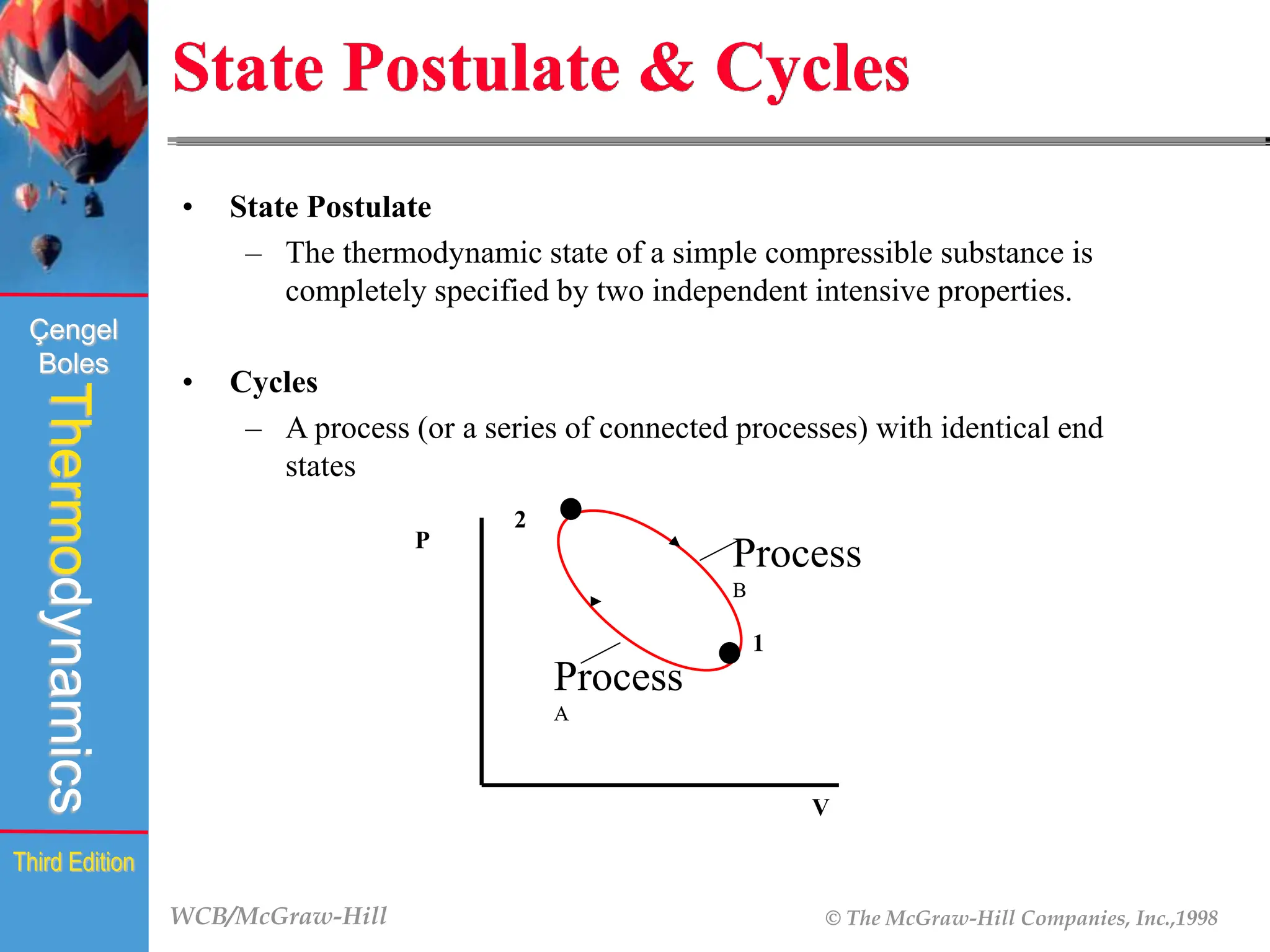
The document discusses the fundamentals of thermodynamics, detailing energy storage and transformation within systems. It explores different approaches to studying thermodynamics, including macroscopic and microscopic views, and describes types of thermodynamic systems such as closed, open, and isolated systems. Key concepts such as energy forms, properties, equilibrium states, and processes like isobaric and isothermal are also introduced.