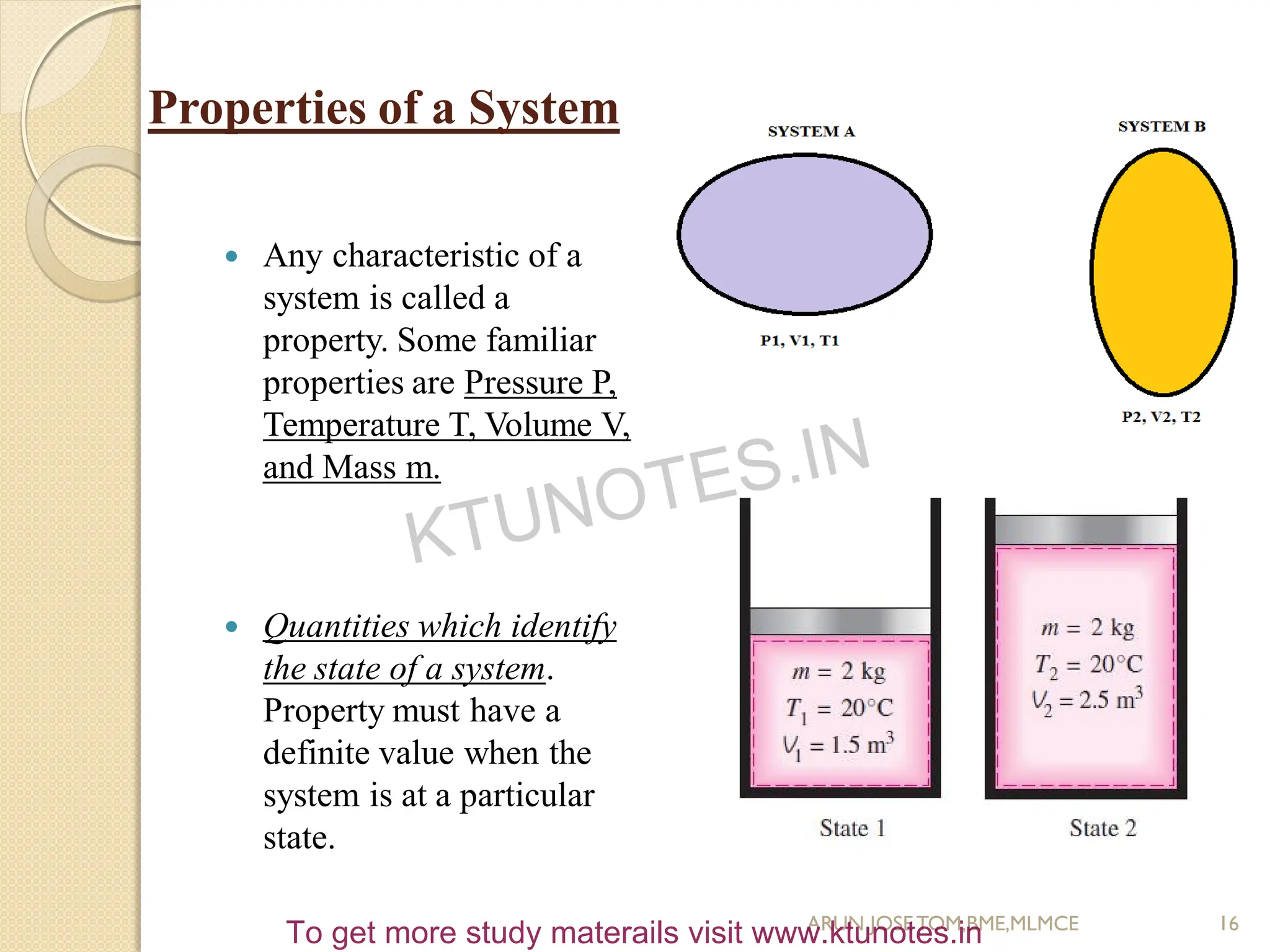
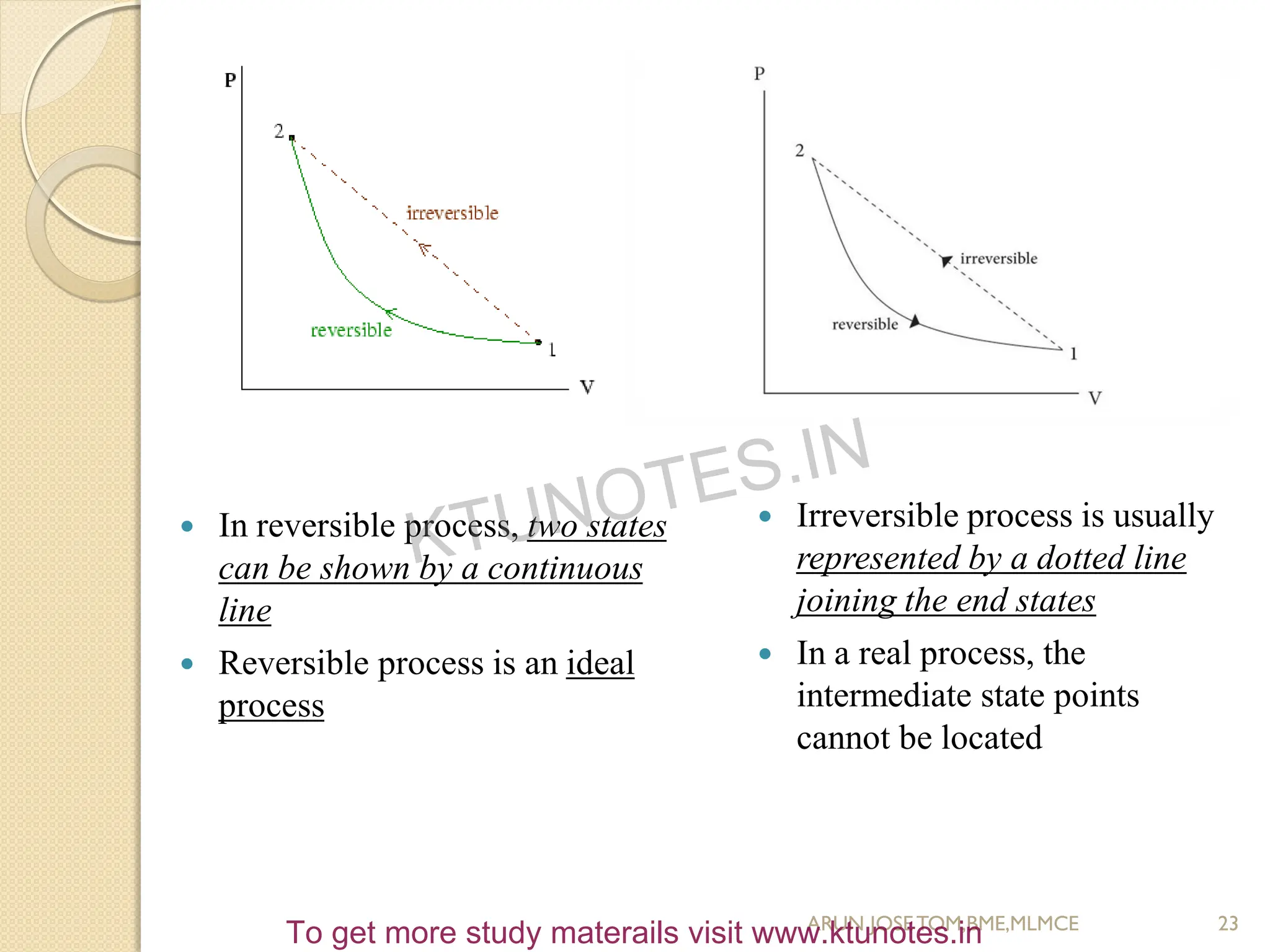
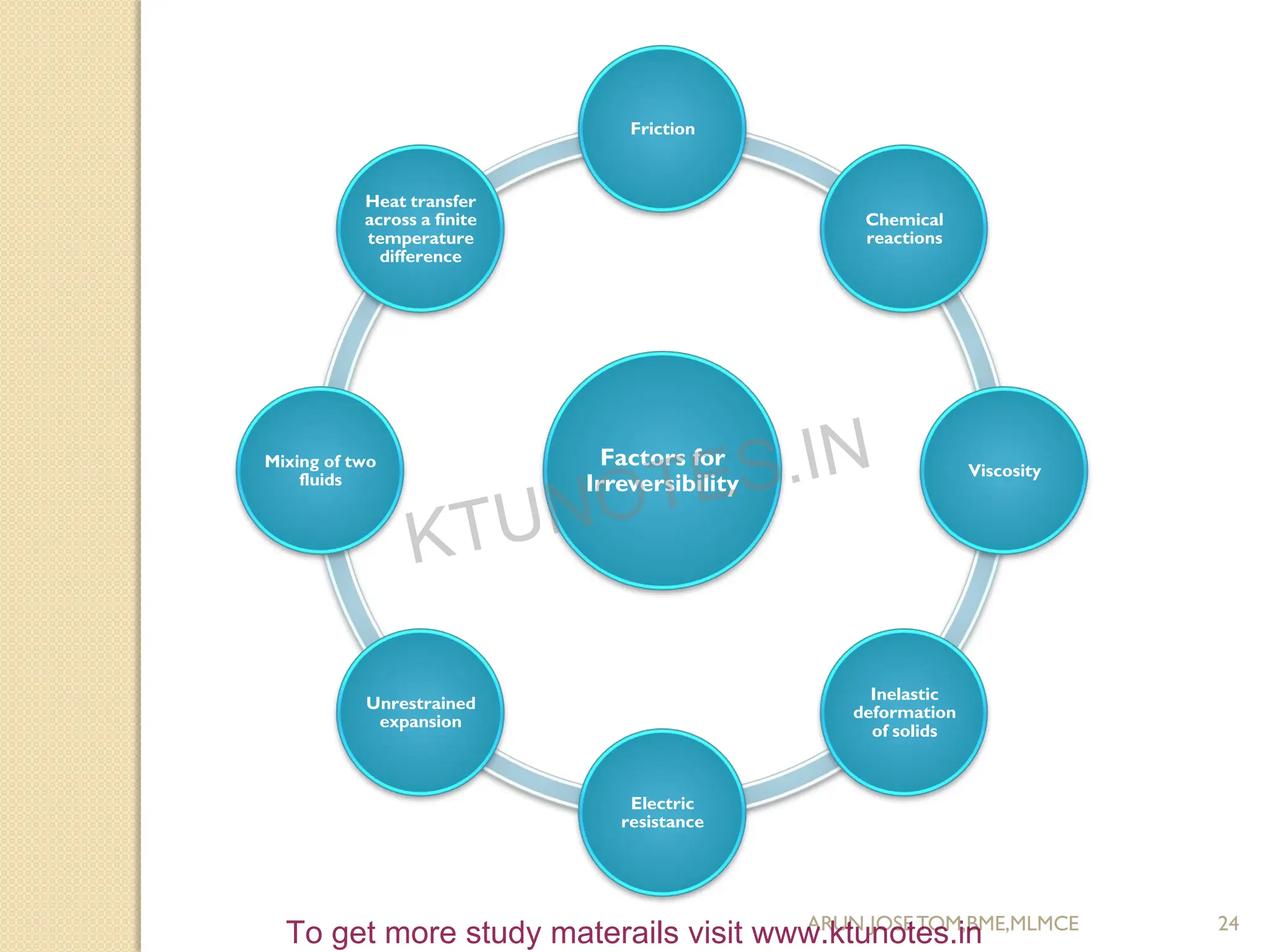
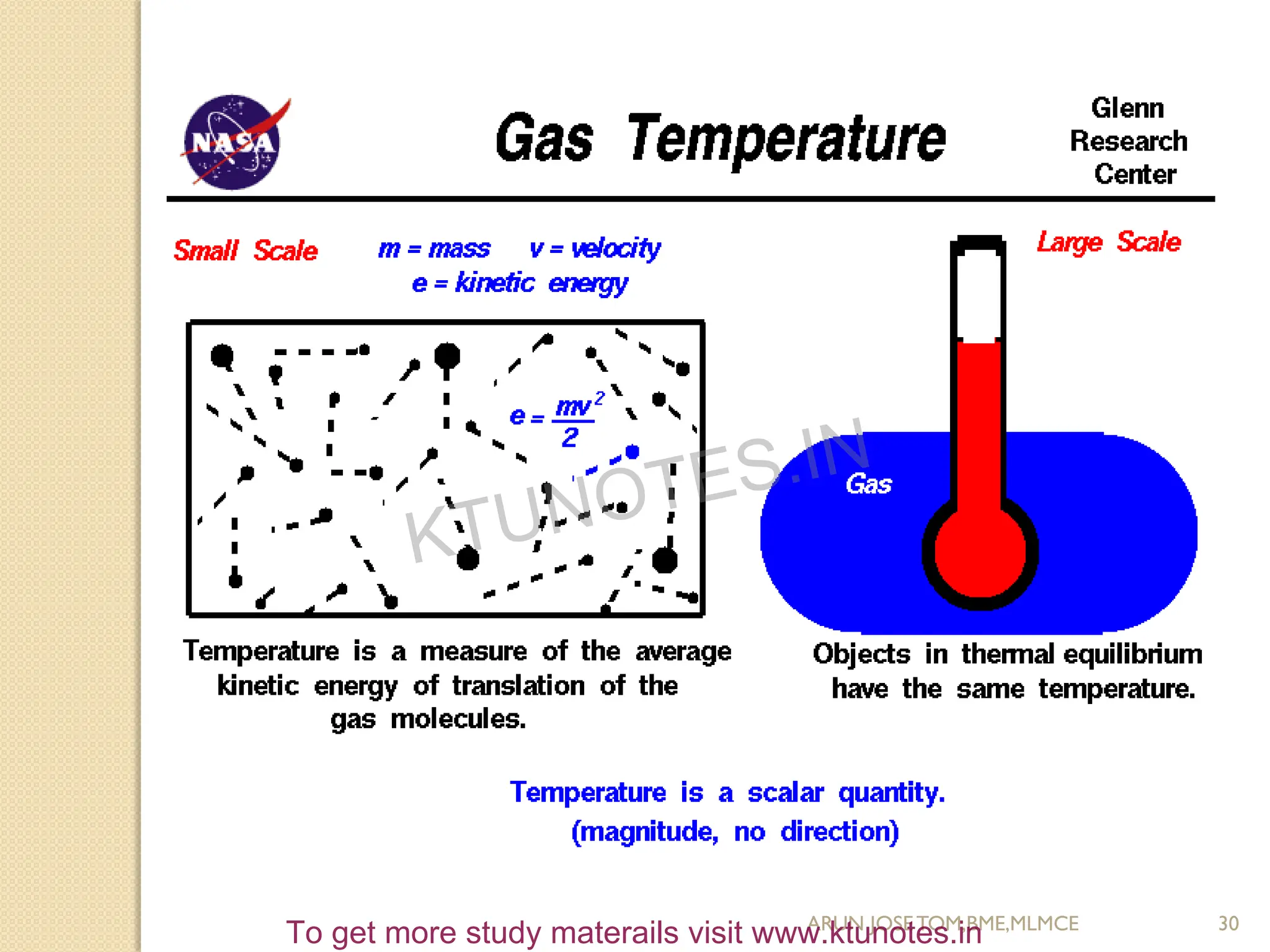
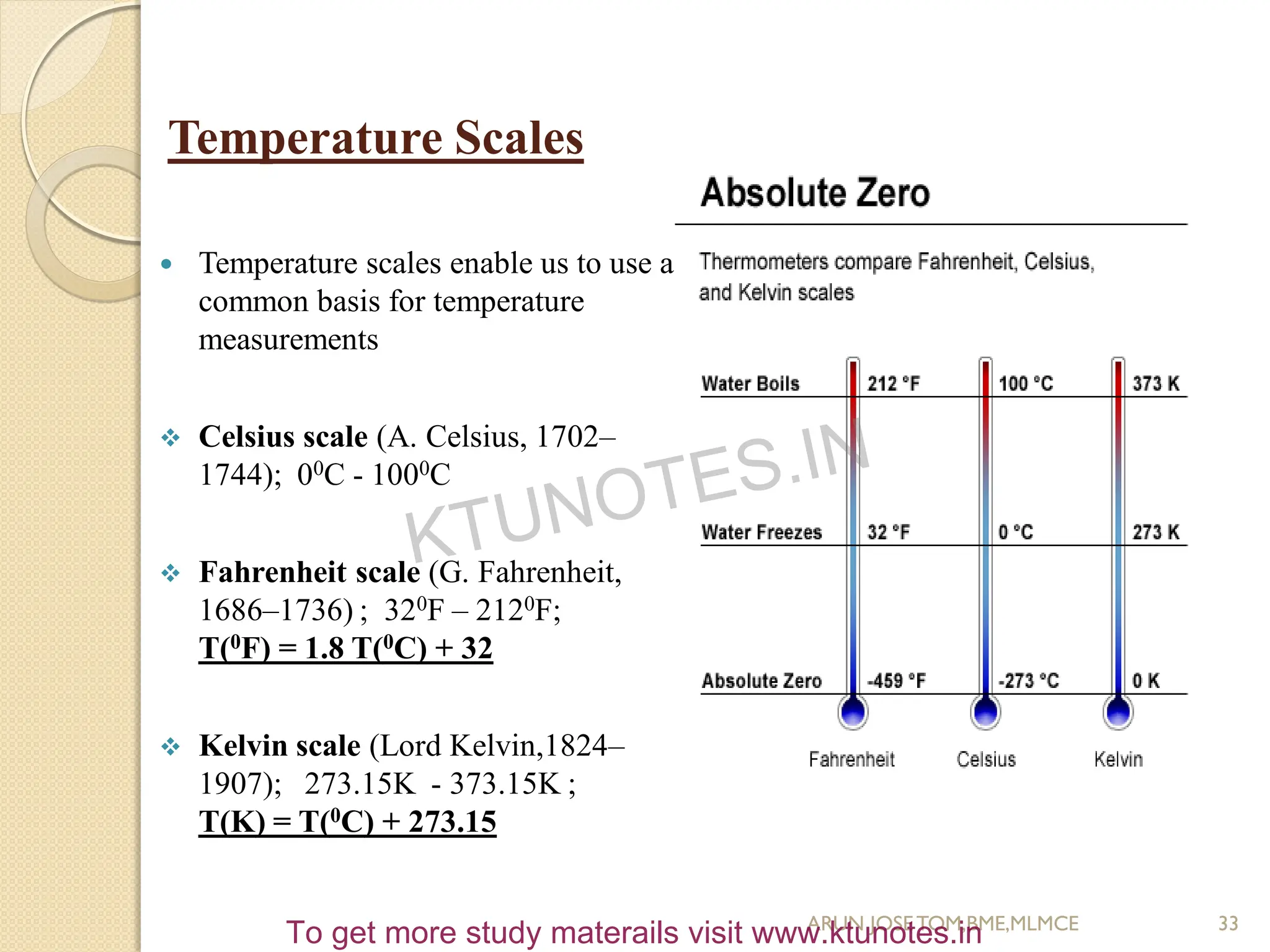
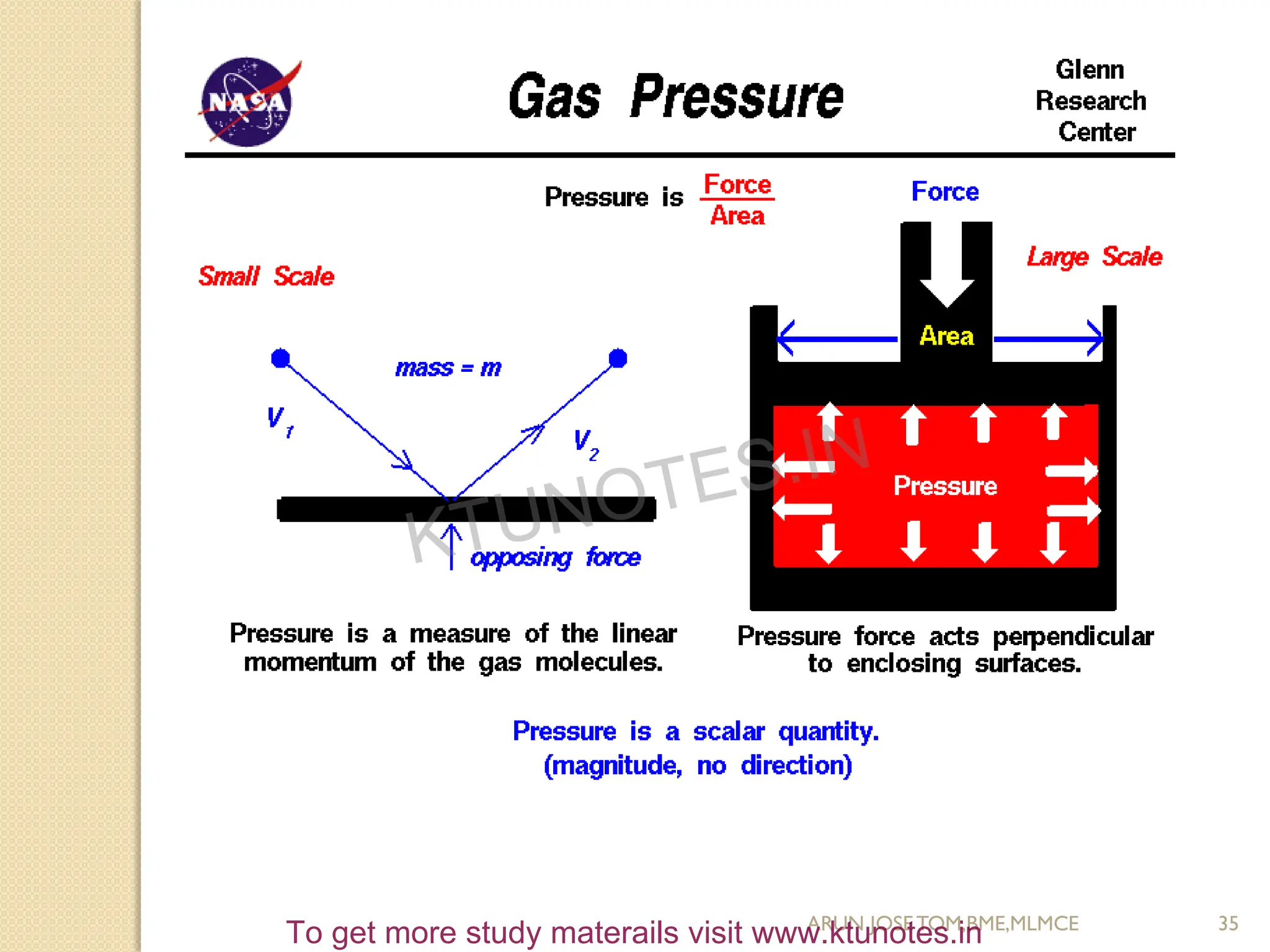
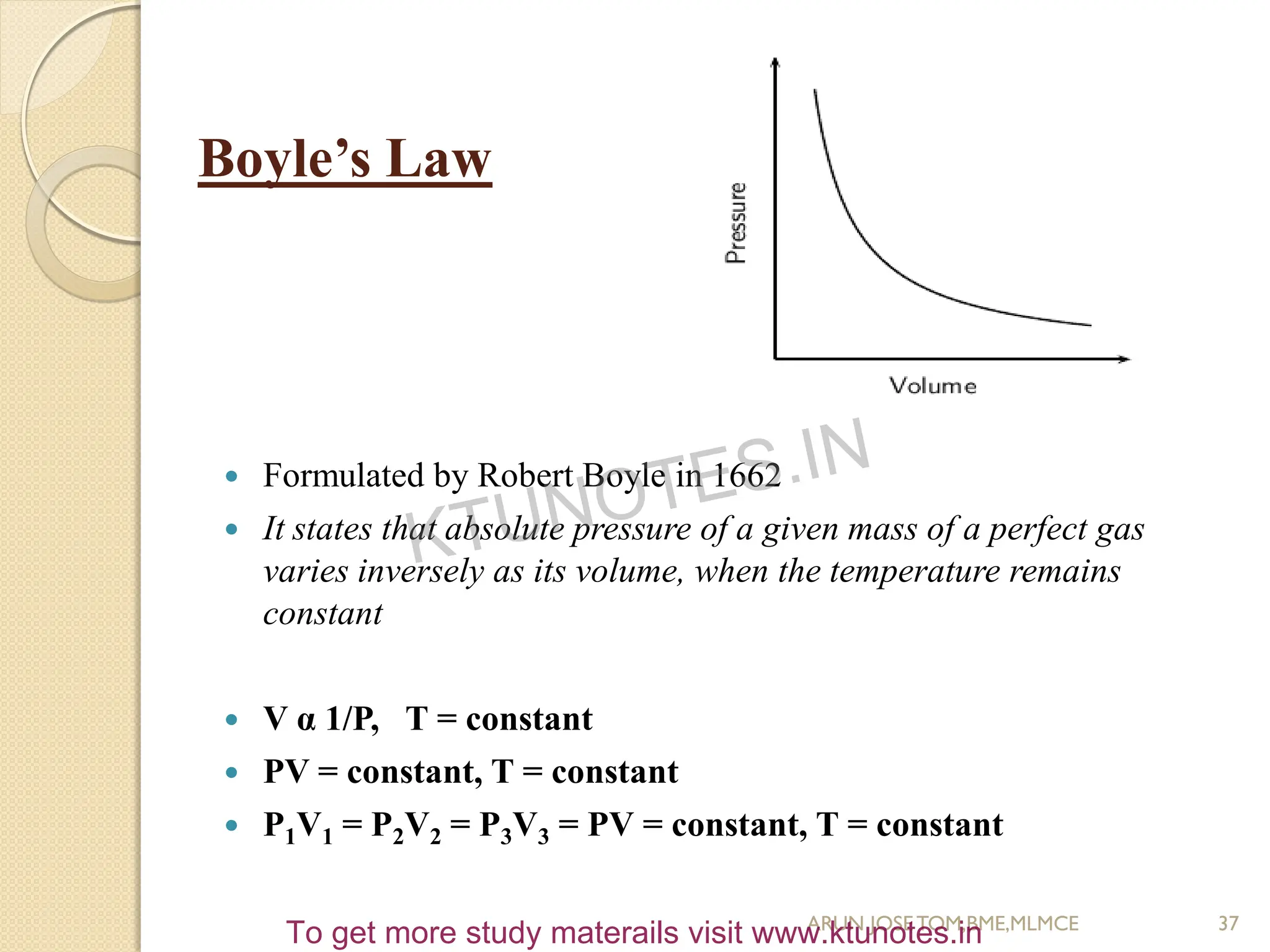
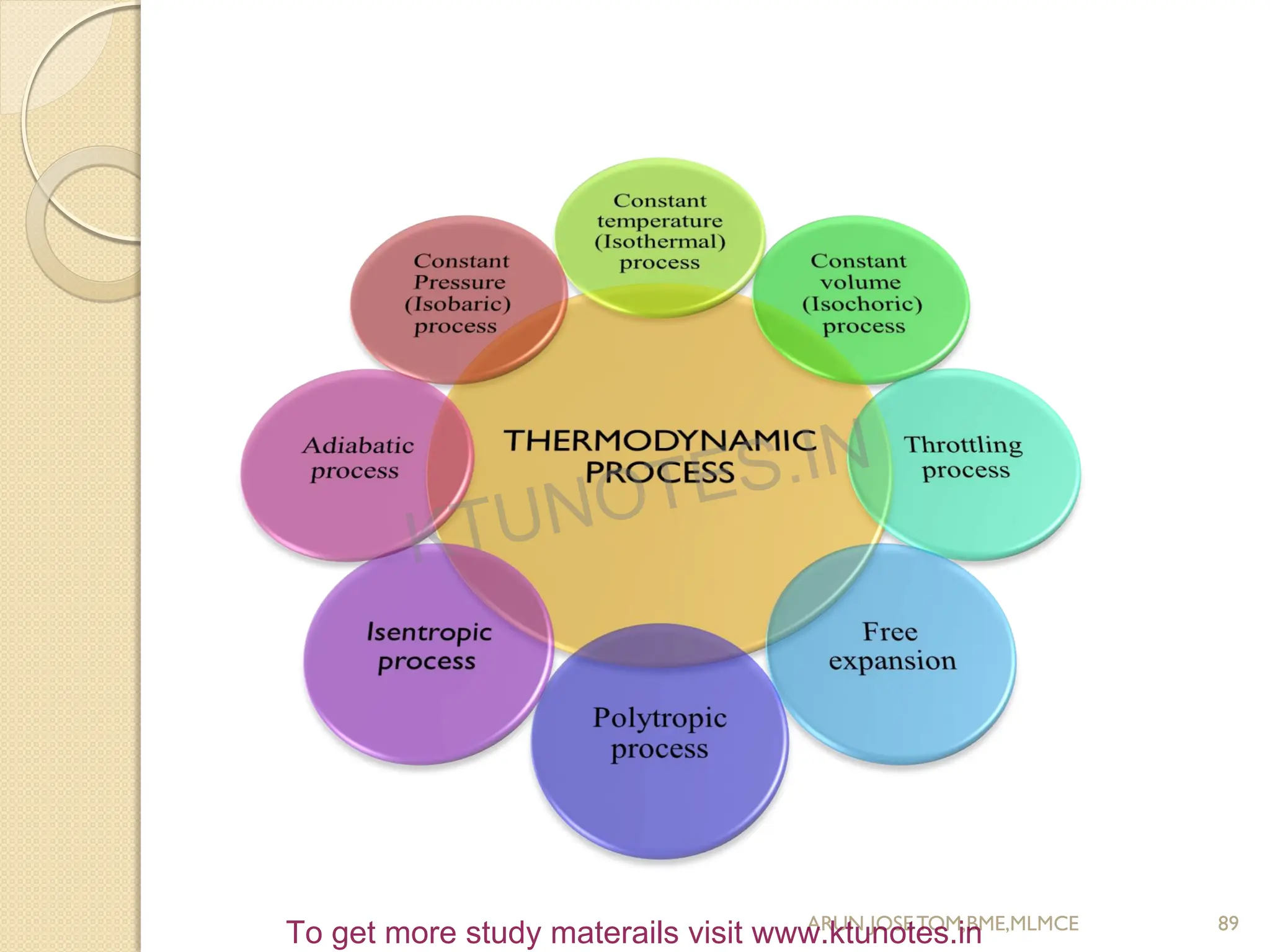
This document provides an introduction to thermodynamics. It begins by stating the objectives of understanding the first and second laws of thermodynamics as well as different thermodynamic processes. It then defines thermodynamics as the science of energy and discusses classical and statistical thermodynamics approaches. The document outlines different types of systems and properties as well as concepts like state, equilibrium, process and path. It introduces various gas laws and the characteristic gas equation.



































![ Heat Capacity, C = Q/dT
Specific heat capacity, c = Q/(mdT), J/Kg K
cp = Q/ [m(T2-T1)]
cv = Q/ [m(T2-T1)]
cp/ cv = γ ; γ- ratio of specific heats
For air, cp= 1.005 KJ/Kg K, cv= 0.718 KJ/Kg K, γ= 1.4
The specific heat at constant pressure Cp is always greater than Cv
because at constant pressure the system is allowed to expand and the
energy for this expansion work must also be supplied to the system
51
ARUN JOSETOM,BME,MLMCE
KTUNOTES.IN
To get more study materails visit www.ktunotes.in](https://image.slidesharecdn.com/bme-m1-ktunotes-240115044335-cc770670/75/BME-51-2048.jpg)
































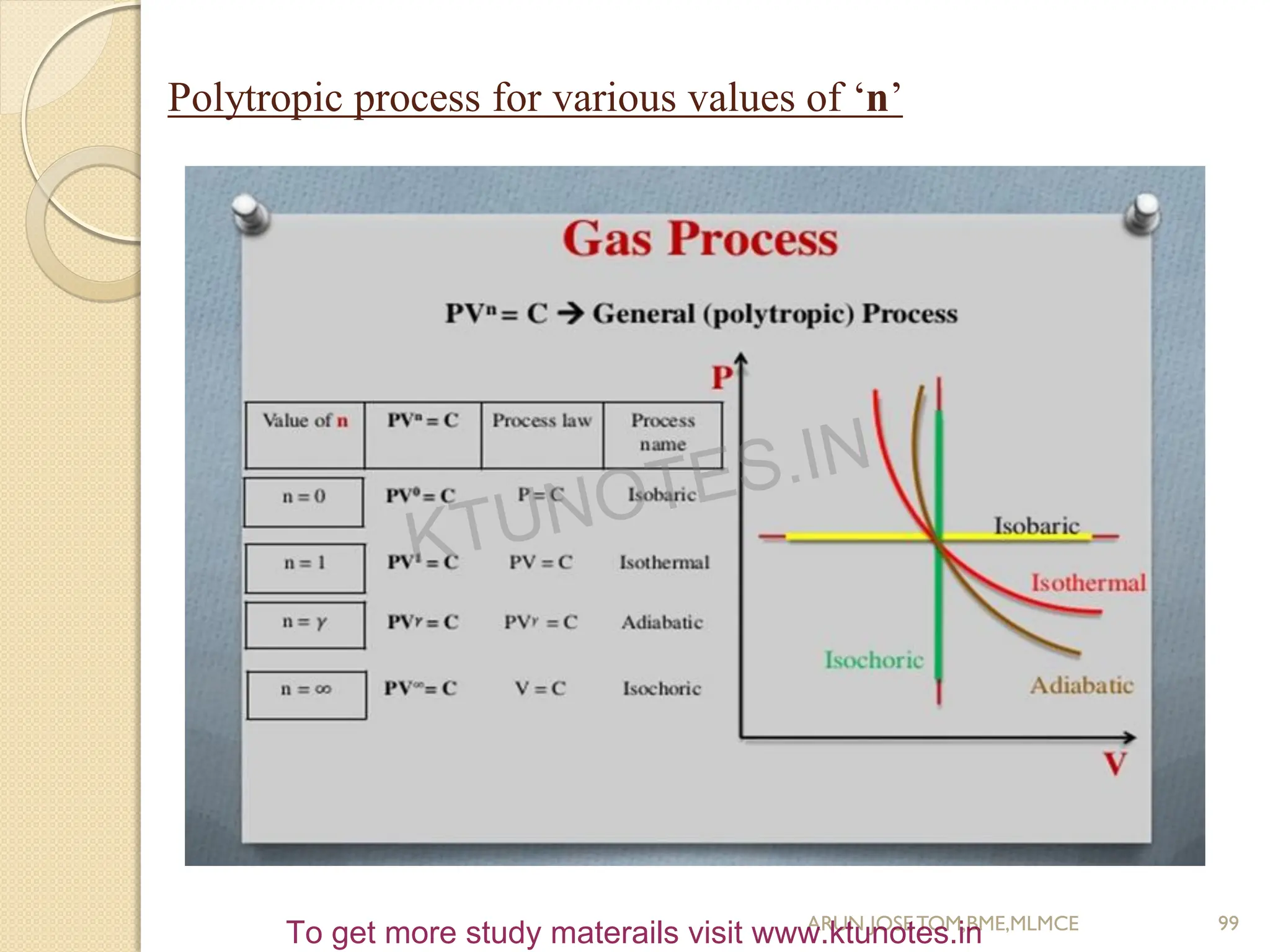
![Polytropic Process
PV
n
= constant
P1V1
n
= P2V2
n
= P3V3
n
= PV
n
= constant
P1/P2 = (V2/V1)
n
= (T1/T2)
n/ n-1
W1-2 = (P1V1 – P2V2)/(n-1)
Q1-2 = [(γ-n)/ (γ-1)] x W1-2
98
ARUN JOSETOM,BME,MLMCE
KTUNOTES.IN
To get more study materails visit www.ktunotes.in](https://image.slidesharecdn.com/bme-m1-ktunotes-240115044335-cc770670/75/BME-98-2048.jpg)







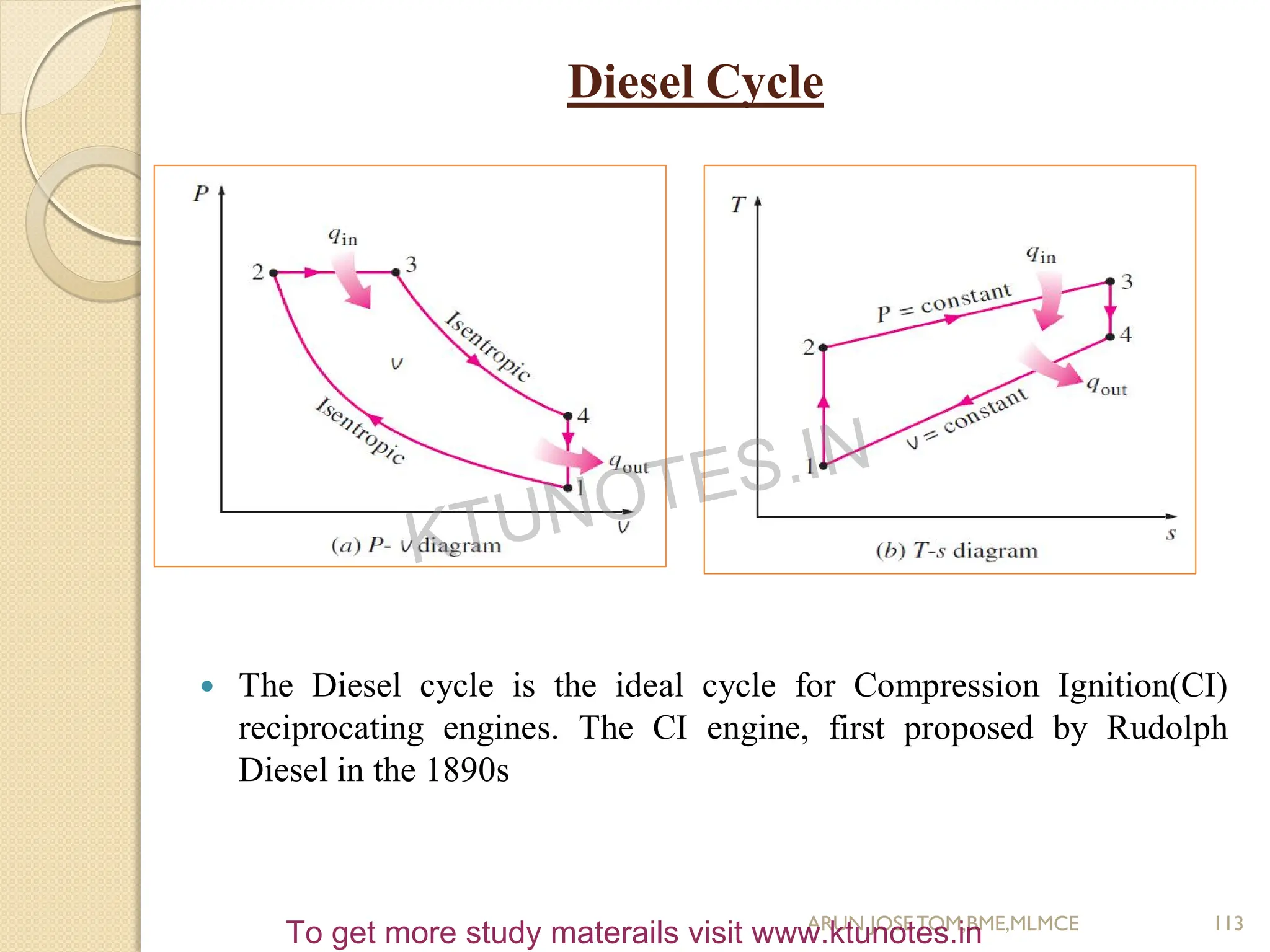
![ Process 1-2, Isentropic compression
Process 2-3, Constant volume heat addition
Process 3-4, Isentropic expansion(Power Stroke)
Process 4-1, Constant volume heat rejection
Compression Ratio, r = V1/V2 ; also Expansion Ratio, r = V4/V3
Heat Supplied, Qin = mcv(T3-T2)
Heat Rejected, Qout = mcv(T4-T1)
Work Done, Wnet = Qin – Qout
Thermal Efficiency, η = 1- (Qout/Qin)
= 1 – [mcv(T4-T1)/ mcv(T3-T2)]
= 1- [1/r
(γ-1)
]
111
ARUN JOSETOM,BME,MLMCE
KTUNOTES.IN
To get more study materails visit www.ktunotes.in](https://image.slidesharecdn.com/bme-m1-ktunotes-240115044335-cc770670/75/BME-111-2048.jpg)


![ Thermal Efficiency, η = 1- (Qout/Qin)
= 1 – [mcv(T4-T1)/ mcp(T3-T2)]
115
ARUN JOSETOM,BME,MLMCE
KTUNOTES.IN
To get more study materails visit www.ktunotes.in](https://image.slidesharecdn.com/bme-m1-ktunotes-240115044335-cc770670/75/BME-115-2048.jpg)