Embed presentation
Download to read offline





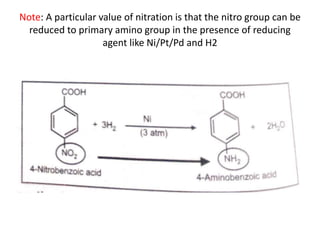
Nitration involves reacting benzene with a nitrating mixture of concentrated nitric acid and concentrated sulfuric acid to produce nitrobenzene. The reaction proceeds in three steps: 1) generation of the electrophilic nitronium ion NO2+ from nitric acid, 2) attack of the nitronium ion on the nucleophilic aromatic benzene ring, and 3) proton transfer to regenerate the aromatic ring structure with a nitro group substituted. A key aspect is that the nitro group can later be reduced to a primary amino group using reducing agents like nickel, platinum, or palladium in the presence of hydrogen gas.





