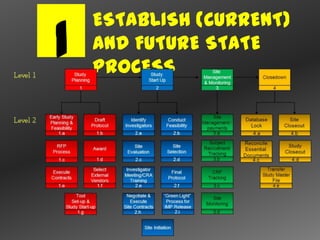
The document outlines a metrics-driven approach to improve the management of clinical trials, focusing on establishing agreed metrics linked to company strategy, understanding performance control, and evaluating the impact of metrics on cost, quality, and time. It emphasizes the importance of training users, mapping metrics to processes, and effectively managing study-specific variations to enhance visibility and insight. Additionally, the document details a structured methodology for conducting site management and interim monitoring, including site visit processes and the development of metrics usage cases and visualizations for performance evaluation.











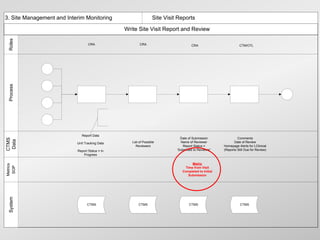
![3. Site Management and Interim Monitoring 3.c. Site Monitoring
3.c.4 [ii] Conduct Interim Visit
Roles
CRA
CRA CRA CRA CRA CRA CRA
ACM
Conduct SDV and Update eCRF and/or CRF Tracking Complete ACM 3.c.4.17
3.c.4(i)
3.c.4.09 Notification Form 3.c.4.18
3.c.4.19
Review Supplies,
Process
Samples and If contact not on CTMS
Facilities
Track and Follow-up
Identify Review Deviations/ Liaise with Site Staff 3.c.4.13 On Site Work
New/Current Staff on Issues.
Deviations/ Violations Against as Significant and/or Completed for
Training Escalate as
Violations Escalation Criteria Urgent Issues Arise IMV
Re-evaluate 3.c.4.15 Necessary
3.c.4.10 3.c.4.11 3.c.4.12 3.c.4.20
Facilities Including 3.c.4.16(i)
Equip. Calibration
and Temp.
3.a.4 Monitoring i.5.1
3.c.4.14
Complete Checklist
Complete Checklist Questions Complete Checklist Questions
Questions Complete Follow-up
CTMS
Track Site and Subject Deviations/ Violations Complete Follow-up Items as Visit
Data
Complete Follow-up Items as Necessary
Track Deviation/Violation Source Data Review Necessary Completed
Items as Necessary Status and Action
Enter Any Additional Relevant Updates Link for any Deviations/ Date
Link for any Deviations/ Taken
Violations
Violations
Metrics
SOP
EDC via
Integration
System
CTMS CTMS CTMS CTMS CTMS CTMS
CTMS](https://image.slidesharecdn.com/nexgenwebinarfeb2013-130306095606-phpapp02/85/Adopting-a-Metrics-Based-Approach-for-Running-Clinical-Trials-Webinar-Feb-2013-13-320.jpg)



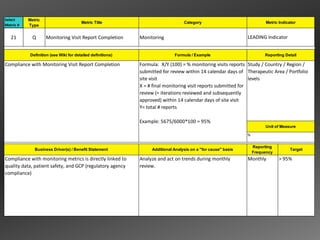


![3. Site Management and Interim Monitoring 3.c. Site Monitoring
3.c.4 [ii] Conduct Interim Visit
Roles
CRA
CRA CRA CRA CRA CRA CRA
ACM
Conduct SDV and Update eCRF and/or CRF Tracking Complete ACM 3.c.4.17
3.c.4(i)
3.c.4.09 Notification Form 3.c.4.18
3.c.4.19
Review Supplies,
Process
Samples and If contact not on CTMS
Facilities
Track and Follow-up
Identify Review Deviations/ Liaise with Site Staff 3.c.4.13 On Site Work
New/Current Staff on Issues.
Deviations/ Violations Against as Significant and/or Completed for
Training Escalate as
Violations Escalation Criteria Urgent Issues Arise IMV
Re-evaluate 3.c.4.15 Necessary
3.c.4.10 3.c.4.11 3.c.4.12 3.c.4.20
Facilities Including 3.c.4.16(i)
Equip. Calibration
and Temp.
3.a.4 Monitoring i.5.1
3.c.4.14
Complete Checklist
Complete Checklist Questions Complete Checklist Questions
Questions Complete Follow-up
CTMS
Track Site and Subject Deviations/ Violations Complete Follow-up Items as Visit
Data
Complete Follow-up Items as Necessary
Track Deviation/Violation Source Data Review Necessary Completed
Items as Necessary Status and Action
Enter Any Additional Relevant Updates Link for any Deviations/ Date
Link for any Deviations/ Taken
Violations
Violations
Metrics
SOP
MCC21
EDC via
Integration
System
CTMS CTMS CTMS CTMS CTMS CTMS
CTMS](https://image.slidesharecdn.com/nexgenwebinarfeb2013-130306095606-phpapp02/85/Adopting-a-Metrics-Based-Approach-for-Running-Clinical-Trials-Webinar-Feb-2013-20-320.jpg)