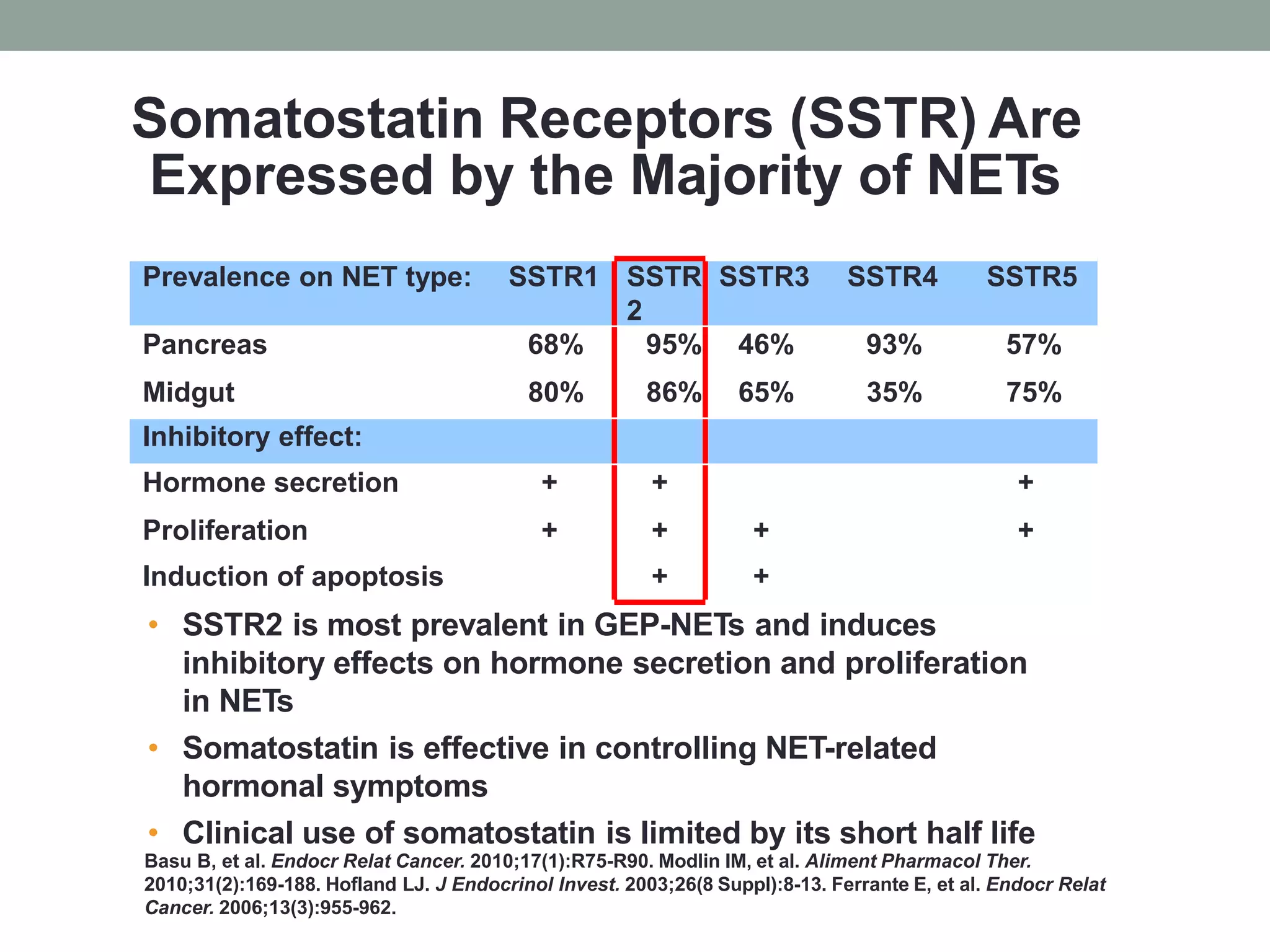
This document discusses neuroendocrine tumors (NETs). It begins with disclosing the speaker's relationships with various pharmaceutical companies. It then outlines some of the challenges in diagnosing and treating NETs, which are rare tumors that can occur in many locations. The document discusses the increasing incidence of NETs and covers their pathology, classification, biomarkers, and imaging. It notes that metastatic disease is common at initial presentation for many patients. Finally, it briefly discusses the goals and options for therapy in advanced NETs, including symptom control and cytotoxic therapy.









































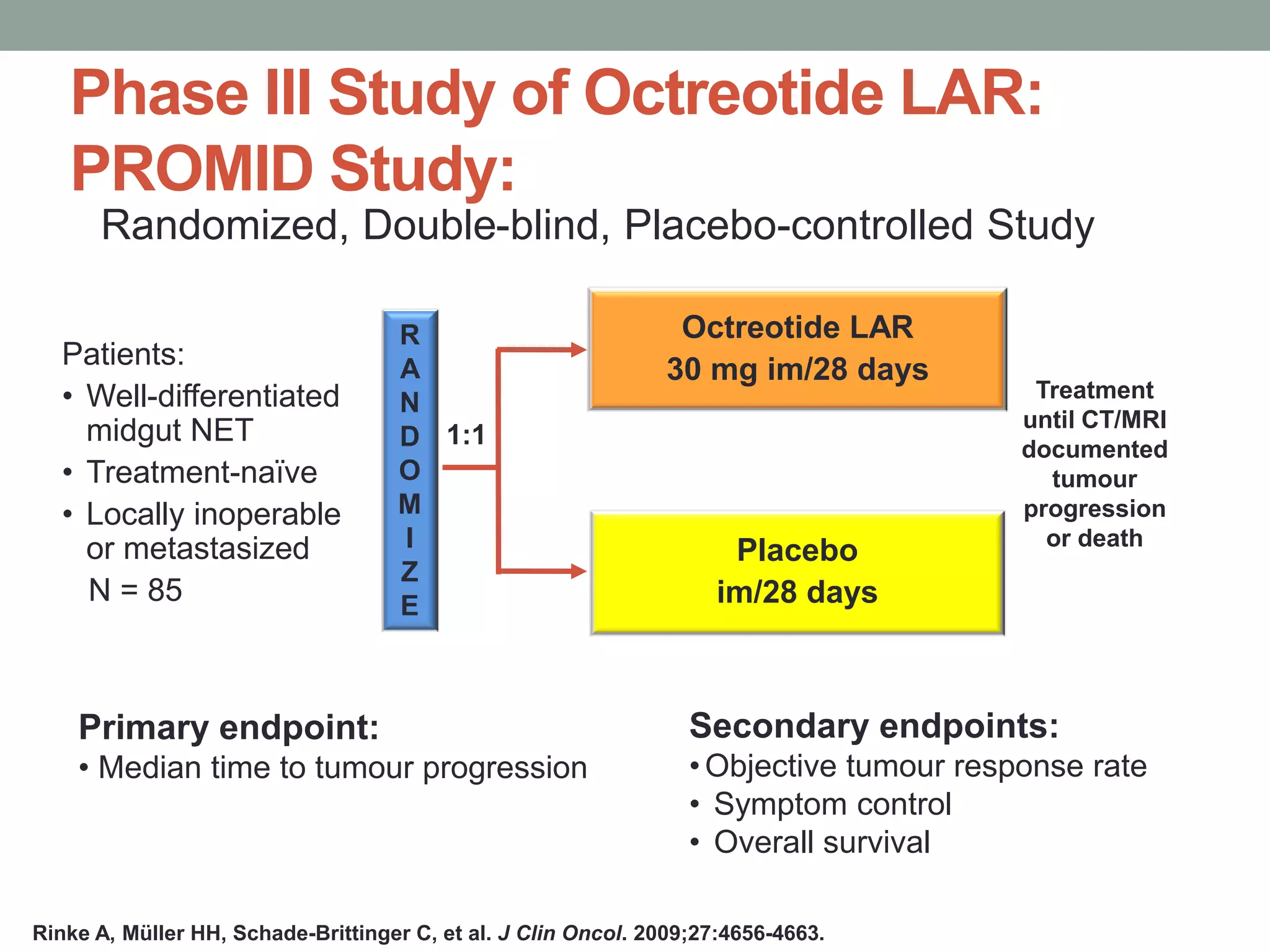
![Octreotide LAR 30 mg
Significantly Prolongs TTP:
HR = hazard ratio. PROMID = Placebo-controlled prospective Randomized study on the antiproliferative efficacy of Octreotide LAR in patients
with metastatic neuroendocrine MIDgut tumours; TTP = time to progression
Rinke A, Müller HH, Schade-Brittinger C, et al. J Clin Oncol. 2009;27:4656-4663.
Octreotide LAR vs placebo
HR=0.34 P=0.000072
[95% CI: 0.20–0.59]
Based on conservative ITT analysis
ProportionWithout
Progression
1.0
.75
.50
.25
0
0 6 12 18 24 30 36 42
Time (mo)
48 54 60 66 72 78
Octreotide LAR (n = 42)
Median 14.3 mo
Placebo (n = 43)
Median 6.0 mo](https://image.slidesharecdn.com/net-novartisluxor2016-160402103147/75/Neuroendocrine-Tumors-in-2016-44-2048.jpg)
















































