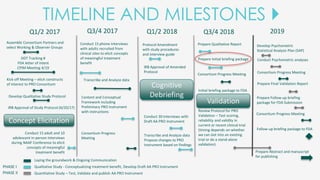
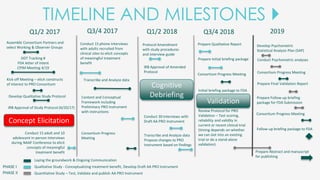
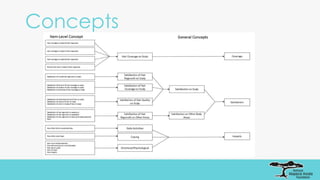
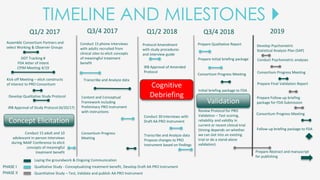
The document outlines the goals of the NAAF Patient-Reported Outcomes Consortium, aiming to develop a standardized, evidence-based patient-reported outcome measure for alopecia areata that can support drug development and regulatory approval. It discusses the consortium's phases, including qualitative studies, cognitive debriefing, and psychometric validation, while highlighting challenges and benefits of collaboration with the FDA and industry partners. The initiative addresses the significant unmet medical need for effective treatment options for alopecia areata by integrating patient voices in the drug development process.