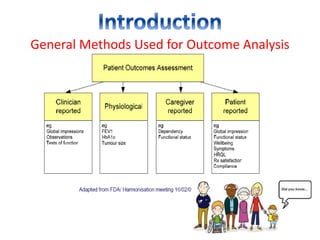
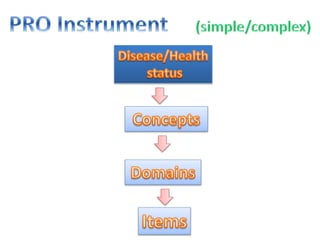
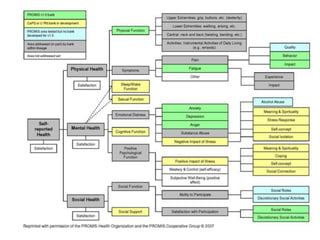
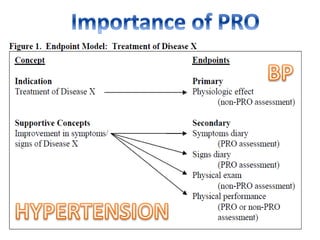
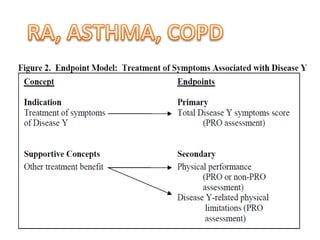
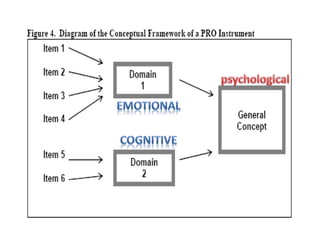
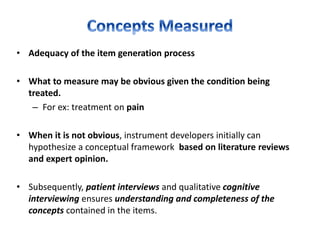
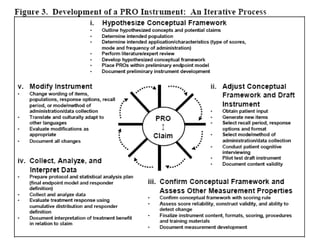
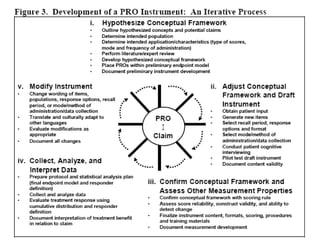
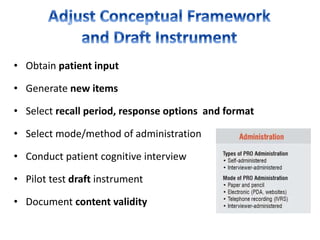
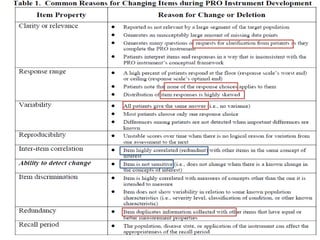
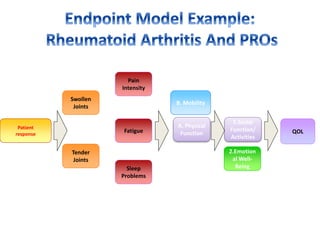
This document discusses patient-reported outcome (PRO) measures and developing PRO instruments. It covers:
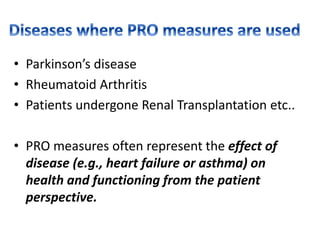
- Common diseases where PROs are used like Parkinson's and rheumatoid arthritis
- Applications of PROs in clinical trials, medical practice, and economic evaluations
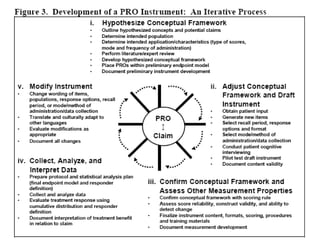
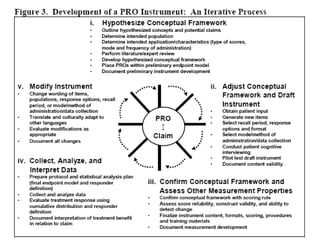
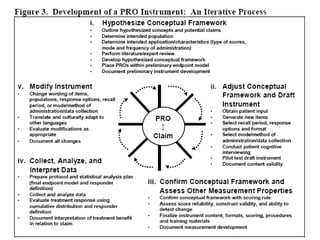
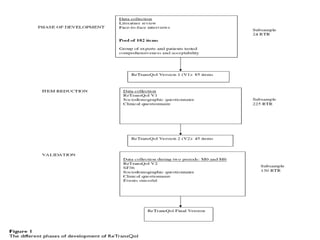
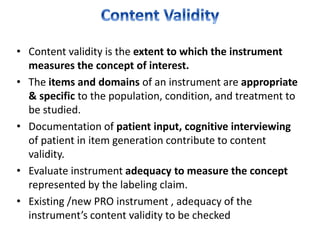
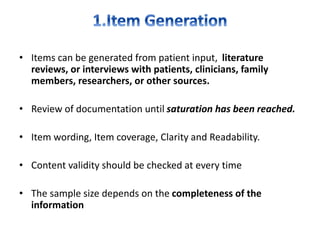
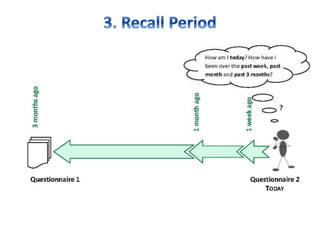
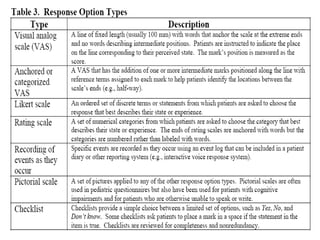
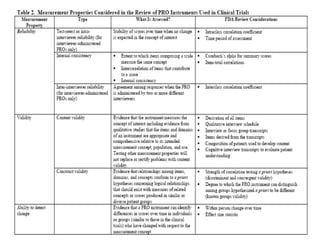
- Developing a conceptual framework, ensuring content validity, and evaluating reliability
- Modifying existing PRO instruments or developing new ones for specific populations like children