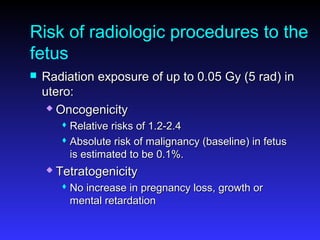
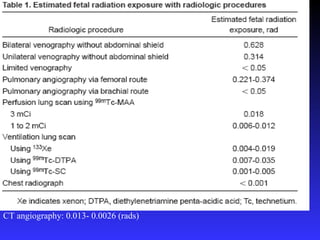
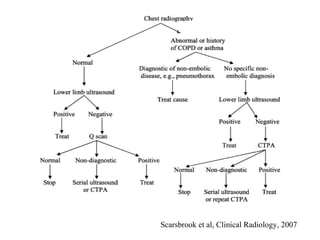
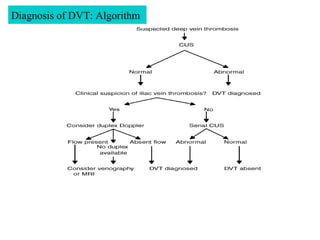
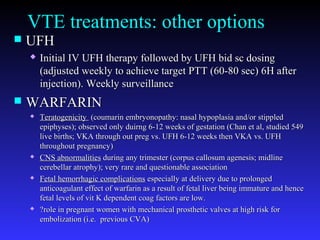
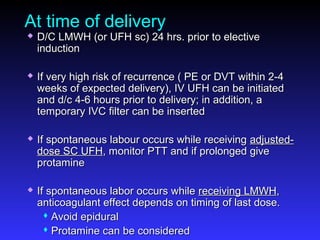
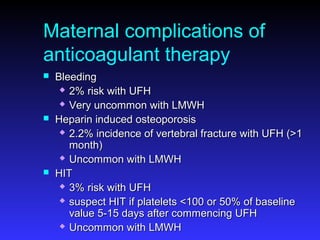
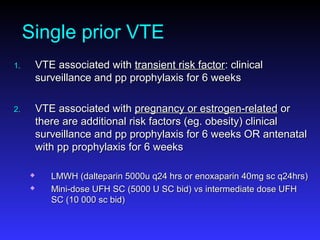
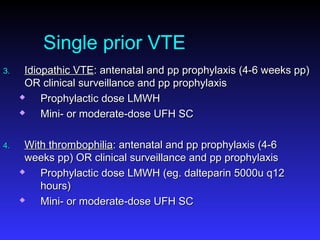
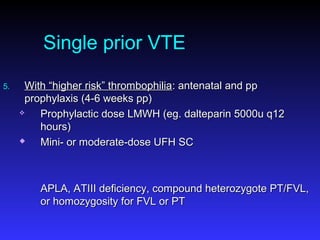
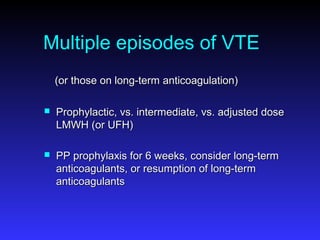
Pregnant women have a 2-4x increased risk of venous thromboembolism (VTE) compared to non-pregnant women. While the left leg is most commonly affected, pelvic and ovarian vein thromboses can also occur. Diagnosis of VTE in pregnancy poses challenges due to atypical symptoms and limitations of diagnostic tests due to radiation exposure risk to the fetus. Low molecular weight heparin is the preferred treatment for VTE in pregnancy due to its safety profile and lack of placental crossing. Untreated VTE can have severe consequences, so diagnosis and treatment are important despite diagnostic limitations.