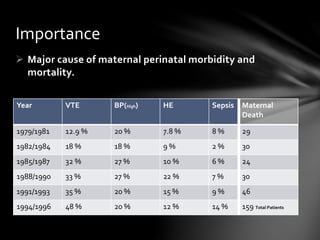
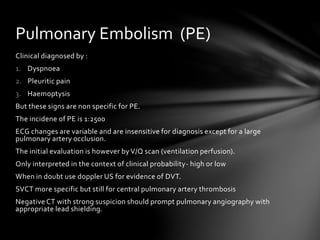
This document discusses venous thromboembolism (VTE), a major cause of maternal morbidity and mortality. It outlines the pathogenesis, risk factors, clinical presentations, diagnostic evaluations, and treatment approaches for various types of VTE that can occur during pregnancy and postpartum, including deep vein thrombosis, pulmonary embolism, pelvic vein thrombosis, and ovarian vein thrombosis. Prevention methods such as pharmacological prophylaxis with heparin and mechanical methods like compression stockings are also summarized.