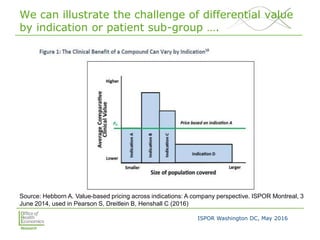
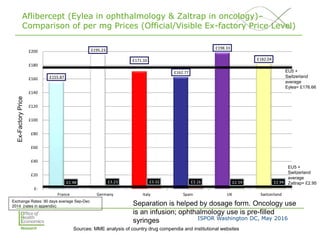
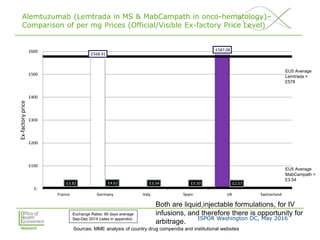
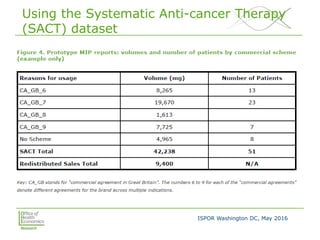
This document summarizes a presentation given at an ISPOR conference on multi-indication pricing. It discusses the challenges of setting one price for a drug across multiple indications when the value may differ based on the indication. It provides examples showing large price differences between indications for the same drugs in different countries. Stakeholders generally support prices reflecting relative value but have concerns about implementation. UK workshop participants felt more collaboration would be needed between stakeholders if the UK pursued multi-indication pricing schemes.