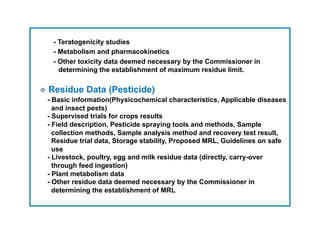
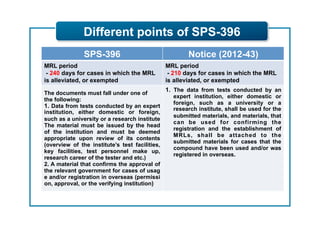
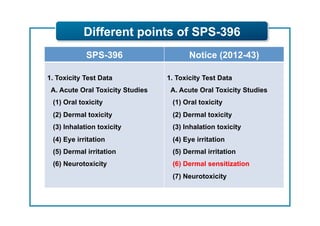
This document outlines guidelines for establishing maximum residue levels (MRLs) of pesticides and veterinary drugs in food in Korea. It describes the scope and procedures for establishing MRLs, including who can apply, what data must be submitted, and how applications will be reviewed by an expert panel. Requirements for toxicity and residue data are provided for both pesticides and veterinary drugs to support MRL applications. The guidelines aim to improve transparency and predictability in the MRL establishment process for companies.