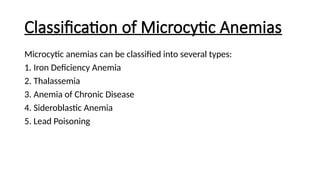
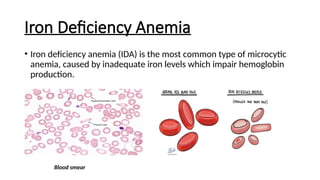
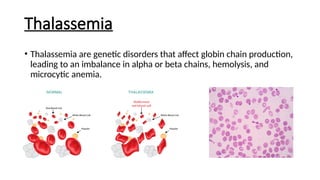
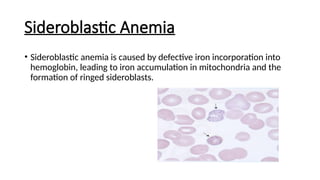
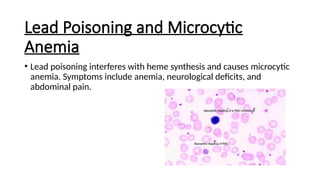
Microcytic anemias are conditions characterized by small red blood cells caused by impaired hemoglobin synthesis, with types including iron deficiency anemia, thalassemia, anemia of chronic disease, sideroblastic anemia, and lead poisoning. Genetic factors play a role in some forms, influencing diagnosis and treatment options. Effective management relies on identifying the specific type and underlying causes of the anemia.