Embed presentation
Download to read offline



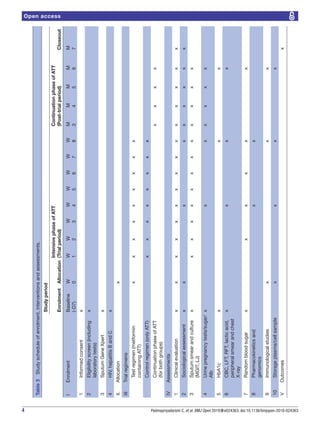




This document presents the study protocol for a randomized clinical trial evaluating the efficacy and safety of adding metformin to standard antituberculosis treatment regimens. The trial aims to determine if metformin can help achieve sputum culture conversion faster when added to initial treatment for drug-sensitive pulmonary tuberculosis. Over 300 participants with newly diagnosed, smear-positive pulmonary TB will be randomized to receive either standard antituberculosis treatment or the same treatment plus metformin for the first two months. The primary outcome is time to sputum culture conversion, with secondary outcomes including time to detection of TB in culture, pharmacokinetic measures, safety, and immune responses. The results could provide evidence for a shorter, more effective TB treatment regimen.







