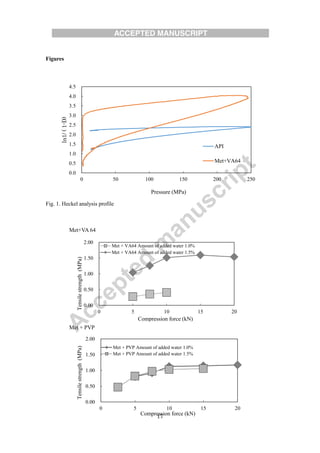
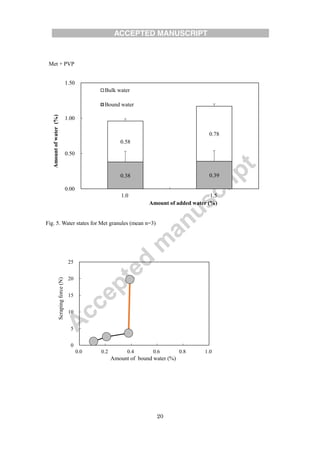
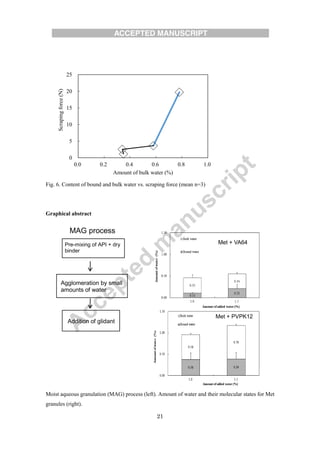
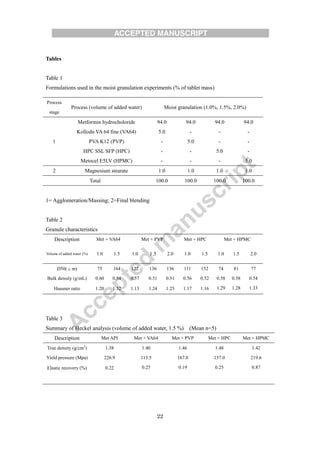
The document evaluates different binders for use in moist aqueous granulation (MAG) formulations of high-dose metformin HCL. Four binders - vinylpyrrolidone-vinyl acetate copolymer, polyvidone, hydroxypropyl cellulose, and hydroxypropyl methylcellulose - were tested. Granules made with hydroxypropyl methylcellulose were not sufficiently granulated with a low water volume. Granules containing hydroxypropyl cellulose had a tendency to form bowls during tableting, while those with polyvidone tended to stick. Vinylpyrrolidone-vinyl acetate copolymer granules maintained proper moisture balance and flow the best. The type of binder used