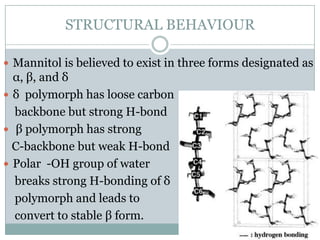
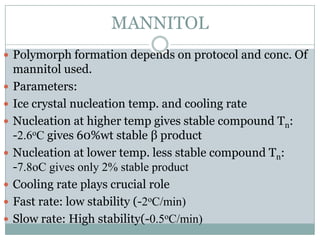
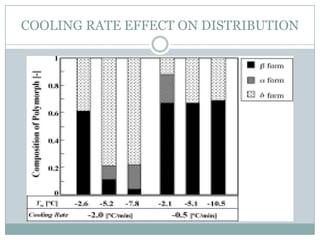
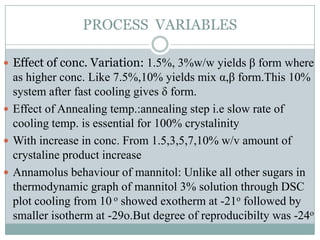
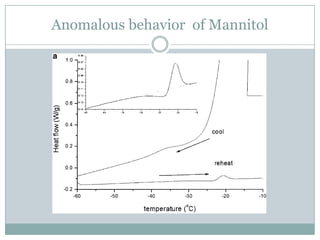
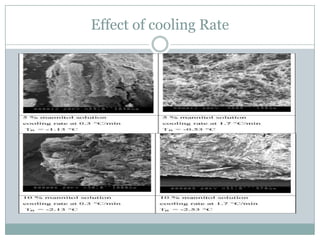
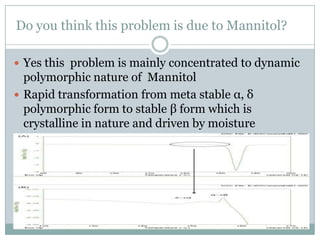
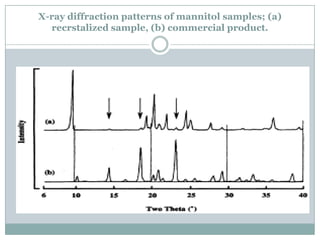
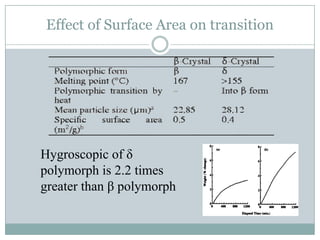
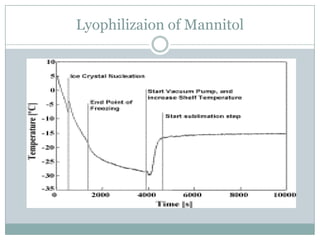
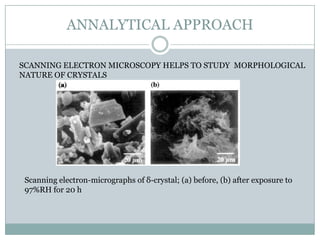
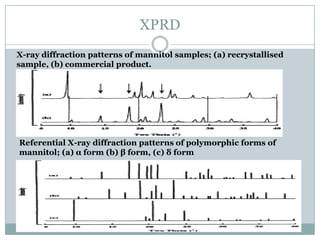
This document summarizes research on the anomalous behavior of mannitol during freeze drying. Mannitol exists in three polymorphic forms (α, β, δ) that have different solubilities and stabilities. The β form has low solubility and is crystalline in nature. Rapid transformation to the stable β form during freeze drying can result in a hazy or turbid solution. Process variables like freezing and annealing rates can affect the resulting polymorph. Approaches to prevent this issue include using a solvent mixture to promote the amorphous δ form, or combining mannitol with other excipients like trehalose that improve stability. Analytical tools like XRD and SEM were used to characterize the polymorphic forms.