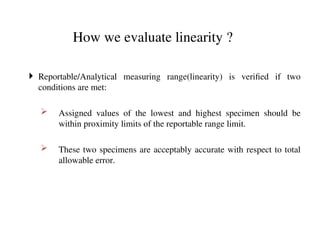
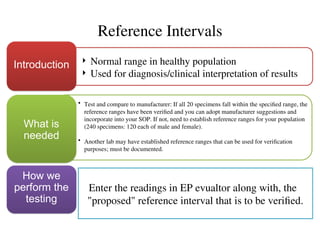
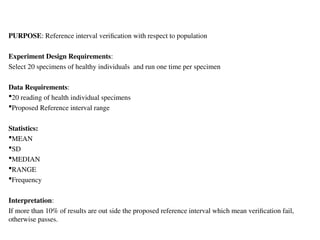
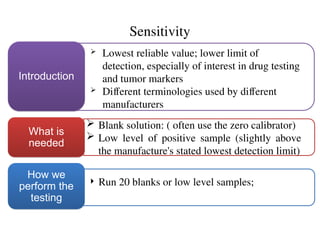
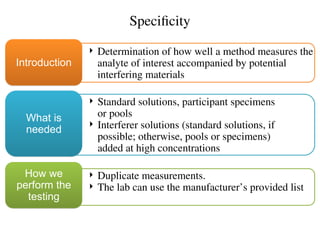
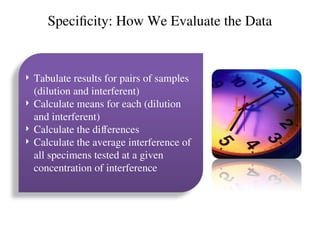
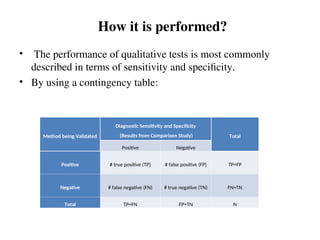
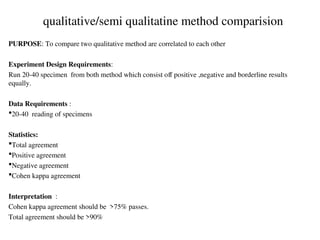
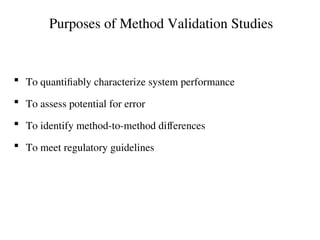
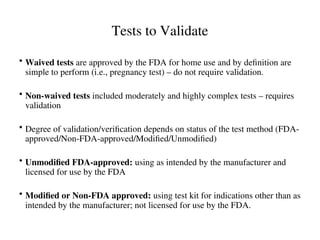
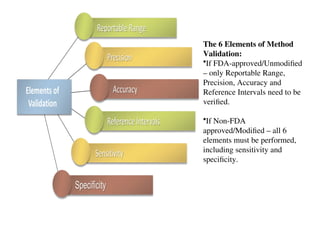
The document outlines the requirements and processes for method validation in clinical laboratories as per the College of American Pathologists (CAP). It details the purpose and necessity of validation, elements involved in validating tests, and statistical evaluations needed to ensure methods are accurate and reliable. The document also emphasizes the need for periodic assessments, comparisons of methods, and specific criteria for waived and non-waived tests.

![What is validation?
• “Validation is the process of testing a measurement
procedure to assess its performance and to determine
whether that performance is acceptable.” [CLSI]](https://image.slidesharecdn.com/methodvalidation-241107201918-e3a31724/85/Method-validation-for-calibration-measure-ppt-2-320.jpg)



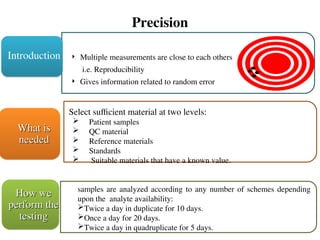

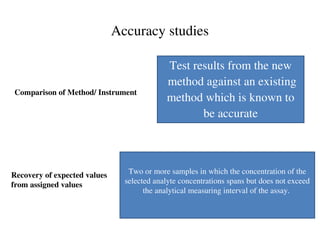
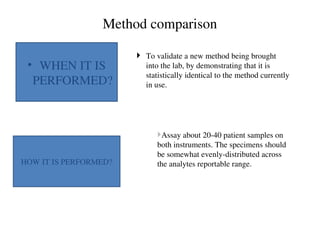
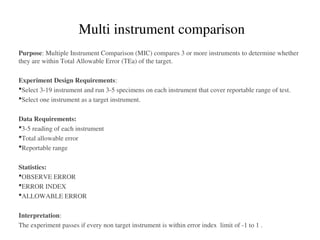
![Accuracy
How close to the true value Comparison of methods
Closeness of the agreement between the result
of a measurement and a true value of the
measurand. [CLSI]
Gives information related to systematic error
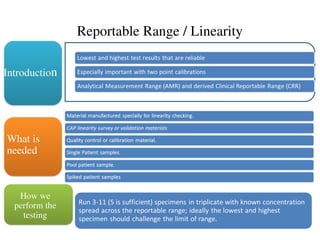
Introduction
40 different specimens
Cover reportable range of method
What is
needed
Duplicate measurements of each specimen
on each method
Minimum of five days, prefer over 20
(since replicate testing is same)
How we
perform the
testing](https://image.slidesharecdn.com/methodvalidation-241107201918-e3a31724/85/Method-validation-for-calibration-measure-ppt-11-320.jpg)