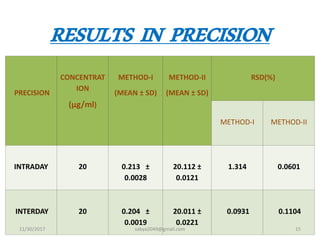
This document details the development and validation of a simple RP-HPLC method for the estimation of sildenafil citrate, a drug used primarily for treating erectile dysfunction, in pharmaceutical formulations. The method was validated according to ICH guidelines, showing high linearity, accuracy, and specificity with no interference from excipients. Results indicate that this method can be accurately used for routine analysis of sildenafil citrate in both bulk and tablet forms.

![INTRODUCTION
Sildenafil Citrate is 5-[2-ethoxy-5- (4-
methylpiperazin-1-ylsulfonyl)phenyl]-1methyl-3-
propyl-1, 6-dihydro-7H-pyrazolo [4, 3-d] pyrimidin-
7-one citrate .
It is used as a smooth muscle relaxtant by enhancing
the effect of NO by inhibiting phosphodiesterase
type 5 (responsible for degradation of cGMP in
corpus cavernosum).
It is used mainly for the treatment of erectile
dysfunction.
11/30/2017 sabya2049@gmail.com 2](https://image.slidesharecdn.com/sabya-171130195742/85/Method-development-and-validation-technique-2-320.jpg)

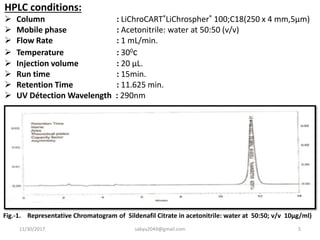
![MATERIALS AND METHODS
• 1] Instrumentation: Quantitative HPLC was
performed on Shimadzu HPLC with LC- 20AT pumps
besides SPD- 20A UV-Visible detector. Shimadzu
Spinchrom-CFR software is used along with inertsil
shield RP-C8 (150 mm × 3.9 mm), 5 µm column for the
separation. A calibrated electronic single pan of Mettler
Toledo was used.
• 2] Reagents Used: HPLC grade acetonitrile,triple
distilled water, pure drug & marketed formulation of
Sildenafil Citrate.
11/30/2017 sabya2049@gmail.com 4](https://image.slidesharecdn.com/sabya-171130195742/85/Method-development-and-validation-technique-4-320.jpg)



![Chemical data:
Chemical name : 5-[2-ethoxy-5- (4-methylpiperazin-1-
ylsulfonyl)phenyl]-1-methyl-3-propyl-1,6-
dihydro-7H-pyrazolo[4,3-d] pyrimidin-7-one
citrate
Empirical formula : C22H30N6O4S, C6H8O7
Molecular weight : 666.7 gm/mole
Physical stability:
Colour : White to off-white.
Solubility : Soluble in dimethylformamide, sparingly
soluble in acetic acid, slightly soluble in methanol.
Form : Crystalline powder
Usual strengths : 25mg;50mg;100mg
11/30/2017 sabya2049@gmail.com 10](https://image.slidesharecdn.com/sabya-171130195742/85/Method-development-and-validation-technique-10-320.jpg)