The “Immunosurge” Continues: Moving in Leaps and Bounds to Expand the Role and Impact of Immunotherapy in Metastatic, Locally Advanced, and Early-Stage NSCLC
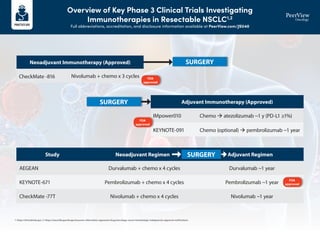
- 1. Overview of Key Phase 3 Clinical Trials Investigating Immunotherapies in Resectable NSCLC1,2 Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40 1. https://clinicaltrials.gov. 2. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications. SURGERY SURGERY Neoadjuvant Immunotherapy (Approved) Study Neoadjuvant Regimen Adjuvant Regimen Adjuvant Immunotherapy (Approved) AEGEAN Durvalumab + chemo x 4 cycles Durvalumab ~1 year CheckMate -77T Nivolumab + chemo x 4 cycles Nivolumab ~1 year KEYNOTE-671 Pembrolizumab + chemo x 4 cycles Pembrolizumab ~1 year CheckMate -816 IMpower010 KEYNOTE-091 Nivolumab + chemo x 3 cycles Chemo atezolizumab ~1 y (PD-L1 ≥1%) Chemo (optional) pembrolizumab ~1 year FDA approved FDA approved FDA approved SURGERY
- 2. NSCLC Treatment Algorithm1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40 Stage and workup based on stage • cT1abc, N0: PFT, bronch, mediastinal staging, PET • cT2a-4, N0-3, M0-1: PFT, bronch, mediastinal staging, PET, brain MRI, and biomarker/mutation testing Surgical candidate? Lobectomy (preferred) or segmentectomy/ wedge resection (in select cases) SBRT or conventionally fractionated RT Surgical resection Mutational testing (if not conducted earlier) EGFR ex19del/ex21 L858R present? Surgical resection T1 N0 M0 Operable disease Yes Yes Yes No No No Multidisciplinary discussion for neoadjuvant candidacy T1–2, N1–2, M0 T3–4, N0–1, M0 Neoadjuvant chemoimmunotherapy Neoadjuvant portion of perioperative immunotherapy Nivolumab + platinum-based chemotherapy x 3 cycles CheckMate -816: Nivo + chemo vs chemo (see FDA approval) mEFS: 31.6 vs 20.8 mo (HR, 0.63) Pembrolizumab + platinum-containing chemotherapy x 4 cycles as neoadjuvant KEYNOTE-671: Pembro + chemo vs placebo + chemo (neoadjuvant) / pembro vs placebo (adjuvant) (see FDA approval) mOS: not reached vs 52.4 mo; mEFS: not reached vs 17 mo Adjuvant chemotherapy Platinum-based chemotherapy LACE Meta-analysis: 5-y OS improvement of 5.4% vs no chemo Adjuvant immunotherapy (stage II-IIIA) Atezolizumab x 16 cycles Stage II-IIIA, PD-L1 ≥1% (see FDA approval) Atezolizumab x 16 cycles IMpower010: Atezo vs BSC mDFS: NR vs 35.3 mo (HR, 0.66) Pembrolizumab x 1 y Stage IB (T2a ≥4 cm), II, or IIIA, regardless of PD-L1 expression (see FDA approval) Pembrolizumab x 1 y PEARLS/KEYNOTE-091: Pembro vs placebo mDFS: 53.6 vs 42.0 mo (HR, 0.76) Adjuvant targeted therapy Osimertinib x 3 y ADAURA: Osimertinib vs placebo 2-y DFS (stage II-IIIA): 90% vs 44% (HR, 0.17) NSCLC treatment algorithm Stage IB-IIIA (resectable) Stage IA Surgical resection Adjuvant portion of perioperative immunotherapy Pembrolizumab, up to 13 cycles KEYNOTE-671: Pembro + chemo vs placebo + chemo (neoadjuvant) / pembro vs placebo (adjuvant) (see FDA approval) mOS: not reached vs 52.4 mo; mEFS: not reached vs 17 mo Yes
- 3. Stage IIIA (unresectable) or IIIB/C Definitive chemoradiation → durvalumab Concurrent platinum-based chemotherapy and radiation with consolidation durvalumab PACIFIC: Durvalumab vs placebo mPFS: 16.8 vs 5.6 mo (HR, 0.52) BRAF V600E Dabrafenib + trametiniba BRF113928: Dabrafenib + trametinib single arm ORR: 64% (95% CI, 46-79) Encorafenib + binimetiniba PHAROS: Encorafenib + binimetinib single arm (see FDA approval) Treatment-naïve patients, ORR: 75% (95% CI, 62-85); mDOR: NE (95% CI, 23.1-NE) Previously treated patients, ORR: 46% (95% CI, 30-63); mDOR: 16.7 mo (95% CI, 7.4-NE) Vemurafenib AcSé: Vemurafenib single arm ORR: 45%; mPFS: 5.2 mo; OS: 10 mo 2L: KRAS G12C Sotorasib CodeBreaK100: Sotorasib single arm ORR: 37.1% (95% CI, 29-46); mPFS: 6.8 mo Adagrasib KRYSTAL-1: Adagrasib single arm (see FDA approval) ORR: 43% (95% CI, 34-53); mDOR: 8.5 mo ALK Alectiniba ALEX: Alectinib vs crizotinib 1-y PFS: 68.4% vs 48.7% (HR, 0.47) Brigatiniba ALTA-1L: Brigatinib vs crizotinib mPFS: 24 vs 11.1 mo (HR, 0.48) Lorlatiniba CROWN: Lorlatinib vs crizotinib mPFS: NR vs 9.3 mo (HR, 0.28); 1-y PFS: 78% vs 39% Ceritinib ASCEND-4: Ceritinib vs chemo mPFS: 16.6 vs 8.1 mo (HR, 0.55) Crizotinib PROFILE 1007: Crizotinib vs chemo mPFS: 7.7 vs 3 mo (HR, 0.49) NTRK Larotrecteniba NCT02122913/SCOUT/NAVIGATE: Larotrectenib single arm (see FDA approval) ORR: 75% according to independent review and 80% according to investigator assessment Entrectiniba ALKA/STARTRK: Entrectinib single arm (see FDA approval) ORR: 70% (NSCLC) RET EGFR S768I, L861Q, and/or G719X Selpercatiniba LIBRETTO-001: Selpercatinib single arm (see FDA approval) ORR: 64%; mDOR: 17.5 mo Pralsetiniba ARROW: Pralsetinib single arm (see FDA approval) Treatment-naïve patients, ORR: 78% (95% CI, 68-85); mDOR: 13.4 mo (95% CI, 9.4-23.1) Previously treated patients, ORR: 63% (95% CI, 54-71); mDOR: 38.8 mo (95% CI, 14.8-NE) Cabozantinib NCT01639508: Cabozantinib single arm ORR: 28% Osimertiniba FLAURA: Osimertinib vs erlotinib/gefitinib mPFS: 18.9 vs 10.2 mo (HR, 0.46) Erlotinib EURTAC: Erlotinib vs chemo mPFS: 9.7 vs 5.2 mo (HR, 0.37) Afatiniba LUX-Lung 3: Afatinib vs cis/pemetrexed mPFS: 13.6 vs 6.9 mo (HR, 0.47) Gefitinib IFUM: Gefitinib single arm mPFS: 9.7 mo Dacomitinib ARCHER 1050: Dacomitinib vs gefitinib mOS: 34.1 vs 27 mo (HR, 0.75) Amivantamab + carboplatin + pemetrexed (nonsquamous) MARIPOSA-2: Amivantamb + chemo ± lazertinib vs chemo mPFS: 6.3, 8.3 vs 4.2 mo (HR, 0.48, 0.44); ORR: 64%, 63% vs 36% (P .001 for both) EGFR (ex20) Amivantamab CHRYSALIS: Amivantamab single arm CBR: 74% (95%CI, 63-83); mPFS: 8.3 mo Amivantamb + carboplatin + pemetrexed (nonsquamous)a PAPILLON: Amivantamab + chemo vs chemo mPFS: 11.4 vs 6.7 mo (HR, 0.4); ORR: 73% vs 47% ROS1 Crizotiniba PROFILE 1001: Crizotinib single arm ORR: 72% (95% CI, 58-84) Entrectiniba ALKA STARTRK: Entrectinib single arm ORR: 67.1%; mPFS: 19 mo Ceritinib YONSEI: Ceritinib single arm ORR: 67% (95% CI, 48-81) Repotrectiniba TRIDENT-1: Repotrectinib single arm (see FDA approval) ROS1 TKI-naïve patients, ORR: 79% (95% CI, 68-88); mDOR: 34.1 mo (95% CI, 25.6-NE) Prior ROS1 inhibitor, ORR: 38% (95% CI, 25-52); mDOR: 14.8 mo (95% CI, 7.6-NE) Lorlatinib NCT01970865: Lorlatinib single arm ROS TKI-naïve patients, ORR: 62%; Crizotinib pre-treated patients, ORR: 35% EGFR (ex19 del or L858R) Osimertiniba FLAURA: Osimertinib vs erlotinib/gefitinib mPFS: 18.9 vs 10.2 mo (HR, 0.46) Osimertinib + pemetrexed + cisplatin/carboplatin FLAURA2: Osimertinib + chemo vs osimertinib (see FDA approval) mPFS: 25.5 vs 16.7 mo (HR, 0.62) Erlotinib EURTAC: Erlotinib vs chemo mPFS: 9.7 vs 5.2 mo (HR, 0.37) Afatinib LUX-Lung 3: Afatinib vs cis/pemetrexed mPFS: 13.6 vs 6.9 mo (HR, 0.47) Gefitinib IFUM: Gefitinib single arm mPFS: 9.7 mo Dacomitinib ARCHER 1050: Dacomitinib vs gefitinib mOS: 34.1 vs 27 mo (HR, 0.75) Erlotinib + ramucirumab RELAY: Erlotinib + ramucirumab vs erlotinib mPFS: 19.4 vs 12.4 mo (HR, 0.59) Erlotinib + bevacizumab (nonsquamous) ARTEMIS-CTONG1509: Erlotinib + bevacizumab vs erlotinib mPFS: 17.9 vs 11.2 mo (HR, 0.55) Amivantamab + carboplatin + pemetrexed (nonsquamous) MARIPOSA-2: Amivantamb + chemo ± lazertinib vs chemo mPFS: 6.3, 8.3 vs 4.2 mo (HR, 0.48, 0.44); ORR: 64%, 63% vs 36% (P .001 for both) MET (exon 14) Capmatiniba GEOMETRY mono-1: Capmatinib single arm (see FDA approval) mPFS: 12.4 mo; treatment-naïve patients, ORR: 68% (95% CI, 55-80); DOR: 16.6 mo Previously treated patients, ORR: 44% (95% CI, 34-54); DOR: 9.7 mo Tepotiniba VISION: Tepotinib single arm mPFS: 8.5-11 mo Crizotinib PROFILE 1001: Crizotinib single arm ORR: 32% 2L: HER2 Trastuzumab deruxtecana DESTINY-Lung02: T-DXd 5.4 mg/kg vs 6.4 mg/kg (see FDA approval) ORR: 58% (98% CI, 43-71); mDOR: 8.7 mo (95% CI, 7.1-NE) Trastuzumab emtansine NCT02675829: Trastuzumab emtansine single arm ORR: 44% T1-2, N2–3, M0 T3, N1–3, M0 T4, N0–3, M0 Tx Nx M1 Actionable mutation detected Mutation (broad NGS if possible) and PD-L1 testing NSCLC treatment algorithm Stage and workup based on stage • cT1abc, N0: PFT, bronch, mediastinal staging, PET • cT2a-4, N0-3, M0-1: PFT, bronch, mediastinal staging, PET, brain MRI, and biomarker/mutation testing Please see the next page for recommendations if no actionable mutation is detected Stage IV • EGFR • ALK • ROS1 • BRAF V600E • RET • MET (ex14) • HER2 • NTRK1/2/3 • KRAS G12C NSCLC Treatment Algorithm1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40
- 4. a Denotes NCCN-preferred regimens. b For patients who have no contraindications to PD-1 or PD-L1 inhibitors and who have a PS 0-1. 1. Adapted from an algorithm created by Aakash Desai, MBBS, MPH, and Matthew Ho, MD, PhD, with recent updates from NCCN Guidelines Version 2, 2024. Used with permission from the authors. PD-L1 1%b IMMUNOTHERAPY + CHEMOTHERAPY SQUAMOUS: • Pembrolizumab + chemotherapya (carboplatin + paclitaxel/nab-paclitaxel) KEYNOTE-407: Pembro + chemo vs chemo mPFS: 6.4 vs 4.8 mo (HR, 0.56); mOS: 15.9 vs 11.3 mo (HR, 0.64) • Cemiplimab + chemotherapya (paclitaxel + carboplatin/cisplatin) EMPOWER-Lung 3: Cemi + chemo vs chemo (see FDA approval) mOS: 21.9 vs 13 mo (HR, 0.7) NONSQUAMOUS: • Pembrolizumab + chemotherapya (carboplatin/cisplatin + pemetrexed) KEYNOTE-189: Pembro + chemo vs chemo mPFS: 8.8 vs 4.9 mo (HR, 0.52); 12-mo OS: 69% vs 49% (HR, 0.49) • Atezolizumab + chemotherapy (carboplatin + paclitaxel + bevacizumab) IMpower150: Atezo + chemo vs chemo mPFS: 8.3 vs 6.8 mo (HR, 0.62) • Cemiplimab + chemotherapya (carboplatin/cisplatin + pemetrexed) EMPOWER-Lung 3: Cemi + chemo vs chemo (see FDA approval) mOS: 21.9 vs 13 mo (HR, 0.7) • Atezolizumab + chemotherapy (carboplatin + nab-paclitaxel) IMpower130: Atezo + chemo vs chemo mOS: 18.6 vs 13.9 mo (HR, 0.79); mPFS: 7.0 vs 5.5 mo (HR, 0.64) DUAL IMMUNOTHERAPY + CHEMOTHERAPY SQUAMOUS: • Nivolumab + ipilimumab + chemotherapy (paclitaxel/carboplatin) CheckMate -9LA: Nivo/ipi + chemo vs chemo mOS: 14.1 vs 10.7 mo • Durvalumab + tremelimumab + chemotherapy (carboplatin + nab-paclitaxel) POSEIDON: Durva/treme + chemo vs chemo (see FDA approval) mOS: 13.3 vs 11.7 mo (HR, 0.86) • Durvalumab + tremelimumab + chemotherapy (gemcitabine + carboplatin/cisplatin) POSEIDON: Durva/treme + chemo vs chemo (see FDA approval) mOS: 13.3 vs 11.7 mo (HR, 0.86) NONSQUAMOUS: • Nivolumab + ipilimumab + chemotherapy (paclitaxel/carboplatin) CheckMate -9LA: Nivo/ipi + chemo vs chemo mOS: 14.1 vs 10.7 mo • Durvalumab + tremelimumab + chemotherapy (carboplatin + nab - paclitaxel) POSEIDON: Durva/treme + chemo vs chemo (see FDA approval) mOS: 13.3 vs 11.7 mo (HR, 0.86) • Durvalumab + tremelimumab + chemotherapy (pemetrexed + carboplatin/cisplatin) POSEIDON: Durva/treme + chemo vs chemo (see FDA approval) mOS: 13.3 vs 11.7 mo (HR, 0.86) DUAL IMMUNOTHERAPY Nivolumab + ipilimumab CheckMate -227: Nivo/ipi vs chemo mOS: 17.1 vs 14.9 mo DUAL IMMUNOTHERAPY Nivolumab + ipilimumab CheckMate -227: Nivo/ipi vs chemo mOS: 17.1 vs 14.9 mo DUAL IMMUNOTHERAPY + CHEMOTHERAPY Nivolumab + ipilimumab + chemotherapy (pemetrexed + carboplatin/cisplatin) CheckMate -9LA: Nivo/ipi + chemo vs chemo mOS: 14.1 vs 10.7 mo Durvalumab + tremelimumab + chemotherapy (carboplatin + nab-paclitaxel) POSEIDON: Durva/treme + chemo vs chemo (see FDA approval) mOS: 13.3 vs 11.7 mo (HR, 0.86) Durvalumab + tremelimumab + chemotherapy (pemetrexed + carboplatin/cisplatin) POSEIDON: Durva/treme + chemo vs chemo (see FDA approval) mOS: 13.3 vs 11.7 mo (HR, 0.86) IMMUNOTHERAPY MONOTHERAPY Pembrolizumab KEYNOTE-042: Pembro vs plat-based chemo mOS: 16.7 vs 12.1 mo (HR, 0.81) DUAL IMMUNOTHERAPY Nivolumab + ipilimumab CheckMate -227: Nivo/ipi vs chemo mOS: 17.1 vs 14.9 mo DUAL IMMUNOTHERAPY + CHEMOTHERAPY Nivolumab + ipilimumab + chemotherapy (pemetrexed + carboplatin/cisplatin) CheckMate -9LA: Nivo/ipi + chemo vs chemo OS: 14.1 vs 10.7 mo Durvalumab + tremelimumab + chemotherapy (carboplatin + nab-paclitaxel) POSEIDON: Durva/treme + chemo vs chemo (see FDA approval) mOS: 13.3 vs 11.7 mo (HR, 0.86) Durvalumab + tremelimumab + chemotherapy (pemetrexed + carboplatin/ cisplatin) POSEIDON: Durva/treme + chemo vs chemo (see FDA approval) mOS: 13.3 vs 11.7 mo (HR, 0.86) PD-L1 1%-49% IMMUNOTHERAPY + CHEMOTHERAPY SQUAMOUS: • Pembrolizumab + chemotherapya (carboplatin + paclitaxel/nab-paclitaxel) KEYNOTE-407: Pembro + chemo vs chemo mPFS: 6.4 vs 4.8 mo (HR, 0.56); mOS: 15.9 vs 11.3 mo (HR, 0.64) • Cemiplimab + chemotherapya (paclitaxel + carboplatin/cisplatin) EMPOWER-Lung 3: Cemi + chemo vs chemo (see FDA approval) mOS: 21.9 vs 13 mo (HR, 0.7) NONSQUAMOUS: • Pembrolizumab + chemotherapya (carboplatin + pemetrexed) KEYNOTE-189: Pembro + chemo vs chemo mPFS: 8.8 vs 4.9 mo (HR, 0.52); 12-mo OS: 69% vs 49% (HR, 0.49) • Atezolizumab + chemotherapy (carboplatin + paclitaxel + bevacizumab) IMpower150: Atezo + chemo vs chemo mPFS: 8.3 vs 6.8 mo (HR, 0.62) • Cemiplimab + chemotherapya (carboplatin/cisplatin + pemetrexed) EMPOWER-Lung 3: Cemi + chemo vs chemo (see FDA approval) mOS: 21.9 vs 13 mo (HR, 0.7) PD-L1 ≥50% IMMUNOTHERAPY MONOTHERAPY Pembrolizumaba KEYNOTE-024: Pembro vs platinum-based chemo mPFS: 10.3 vs 6 mo (HR, 0.50) Atezolizumaba IMpower110: Atezo vs platinum-based chemo mOS: 20.1 vs 13.1 mo (HR, 0.59) Cemiplimaba EMPOWER-Lung1: Cemi vs platinum-based chemo mPFS: 8.2 vs 5.7 mo; mOS: NR vs 14.2 mo (HR, 0.57) IMMUNOTHERAPY + CHEMOTHERAPY SQUAMOUS: • Pembrolizumab + chemotherapya (carboplatin + paclitaxel/nab-paclitaxel) KEYNOTE-407: Pembro + chemo vs chemo mPFS: 6.4 vs 4.8 mo (HR, 0.56); mOS: 15.9 vs 11.3 mo (HR, 0.64) • Cemiplimab + chemotherapya (paclitaxel + carboplatin/cisplatin) EMPOWER-Lung 3: Cemi + chemo vs chemo (see FDA approval) mOS: 21.9 vs 13 mo (HR, 0.7) NONSQUAMOUS: • Pembrolizumab + chemotherapya (carboplatin/cisplatin + pemetrexed) KEYNOTE-189: Pembro + chemo vs chemo mPFS: 8.8 vs 4.9 mo (HR, 0.52); 12-mo OS: 69% vs 49% (HR, 0.49) • Atezolizumab + chemotherapy (carboplatin + paclitaxel + bevacizumab) IMpower150: Atezo + chemo vs chemo mPFS: 8.3 vs 6.8 mo (HR, 0.62) • Cemiplimab + chemotherapya (carboplatin/cisplatin + pemetrexed) EMPOWER-Lung 3: Cemi + chemo vs chemo (see FDA approval) mOS: 21.9 vs 13 mo (HR, 0.7) • Cemiplimab + chemotherapy (paclitaxel + carboplatin or cisplatin) EMPOWER-Lung 3: Cemi + chemo vs chemo (see FDA approval) mOS: 21.9 vs 13 mo (HR, 0.7) No actionable mutation detected (stratify based on PD-L1 staining %) NSCLC Treatment Algorithm1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40
- 5. Immune-Related Adverse Events of Cancer Immunotherapies1-17 Become Aware and Stay Vigilant Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40 Immune checkpoint inhibitors (ICIs)—which are monoclonal antibodies against CTLA-4, PD-1, or PD-L1—have transformed treatment of many cancer types. However, in some cases, these treatments are associated with immune-related adverse events (irAEs). MANAGEMENT Treatment of irAEs depends on the affected organ and the severity of symptoms. ICIs should be halted following irAE diagnosis in most patients, except those with very mild symptoms. Glucocorticoids are the first-line therapy for most severe irAEs, following which nonsteroidal synthetic immunosuppressive agents or intravenous immunoglobulin can be used if symptoms do not improve within 48-72 hours. Monoclonal antibody therapy against, for example, TNF or IL-6, or plasma exchange can be used for some irAEs. Deciding when to recommence ICI therapy to continue cancer treatment should be undertaken by a multidisciplinary team comprising organ specialists and oncologists. ICIs should be permanently discontinued in individuals with grade 3 myocarditis, pneumonitis, and hepatitis, among others, and all grade 4 irAEs. OUTLOOK Some studies have identified biomarkers associated with a higher risk of irAEs, such as pretreatment levels of serum autoantibodies. However, further studies are required before these autoantibodies can be used to guide management strategies in clinical practice. Moreover, as new ICIs or new combinations of therapies are approved, studies will be needed to characterize the associated risk, frequency, and manifestations of irAEs. MECHANISMS For CTLA-4 inhibitors, an imbalance in the ratio of regulatory T (Tregs) cells (which dampen the immune response) to type 17 T helper (TH17) cells (which promote the immune response), autoantibody production, and complement-mediated cellular damage have been suggested to contribute to irAE development. The mechanisms underlying PD-1/PD-L1 inhibitor–associated irAEs are less well-understood but could be due to reduced Treg cell numbers. DIAGNOSIS Diagnostic workup of individuals with suspected irAEs depends on the affected organ SYSTEMIC Sicca syndrome and vasculitis irAEs can range in severity and affect almost any organ Polyneuropathy Uveitis Interstitial lung disease Hepatitis Vitiligo Myalgia and myositis Enterocolitis Thyroiditis Hypophysitis Myocarditis Adrenitis Arthralgia and arthritis EPIDEMIOLOGY Onset of irAEs generally occurs between 2 and 16 weeks after ICI initiation, depending on the affected organ; however, some reports have noted onset within a few days of starting therapy and 1 year after completion. In general, PD-1 and PD-L1 inhibitors are tolerated better than CTLA-4 inhibitors, and ICI monotherapy is associated with fewer irAEs than PD-1/PD-L1 and CTLA-4 combination therapy. Pre-existing autoimmune disease is a strong risk factor for developing irAEs ICIs targeting the CTLA-4 or PD-1/PD-L1 pathways facilitate T-cell activation and survival, which induce an antitumor immune response Monitoring organ function during ICI therapy to enable early detection of irAEs is warranted only for some organs, such as thyroid and liver Endocrine irAEs of all severities should be treated with hormone supplementation
- 6. Immune-Related Adverse Events of Cancer Immunotherapies1-17 Become Aware and Stay Vigilant Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40 Patterns and Duration of Various irAEs Pneumonitis: most common fatal toxicity associated with PD-(L)1 monotherapy Myocarditis: most common fatal toxicity associated with PD-(L)1/CTLA-4 combination therapy Endocrinopathies: most common toxicities associated with PD-(L)1 monotherapy Hepatitis: a common toxicity associated with immunotherapy and targeted therapy combinations Cutaneous toxicities: earliest toxicity associated with PD-(L)1 monotherapy and combinations Nephritis: a common toxicity associated with chemo-IO 4 6 8 10 12 14 30 4 6 8 10 12 14 30 4 6 8 10 12 14 30 Duration of Treatment, wk Duration of Treatment, wk CTLA-4 inhibitor PD-1/PD-L1 inhibitor PD-1/PD-L1 + CTLA-4 inhibitors Duration of Treatment, wk Toxicity Grade Toxicity Grade Toxicity Grade Colitis Liver toxicity Skin, rash, or pruritus Pneumonitis Endocrinopathy Nephritis
- 7. Immune-Related Adverse Events of Cancer Immunotherapies1-17 Become Aware and Stay Vigilant Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40 Guidance for Surgeons: Suspect, Detect, and Refer for Treatment • irAEs frequently occur in the perioperative setting, either before or after surgical intervention • irAEs occurring during neoadjuvant immunotherapy are generally manageable and in most cases should not exclude patients from surgery • The onus is on the surgeon to have a high degree of suspicion for potential toxicities in patients treated with immunotherapy • Vague symptoms should not be dismissed; nonspecific ailments can be indicative of severe toxicity – Rheumatologic toxicities and endocrinopathies are some of the most difficult to recognize, given their relatively nonspecific presentation » For example, fatigue, poor energy, and low mood could represent hypophysitis or adrenal insufficiency – Other toxicities can be essentially asymptomatic » For example, renal and hepatic toxicity are generally only detected on routine labs – Pneumonitis is another relevant irAE requiring awareness by surgeons, as severe pneumonitis could potentially exclude patients from operative therapy, but significant pneumonitis has been rare in trials to date • A comprehensive workup for irAEs, with a thorough history specifically targeted to potential irAEs, should be conducted • Coordinate and collaborate with oncologists and other multidisciplinary experts to optimally diagnose and manage irAEs in patients who have received/are receiving perioperative immunotherapy • The National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) have issued guidelines for recognition and management of immune-related adverse events
- 8. Immune-Related Adverse Events of Cancer Immunotherapies1-17 Become Aware and Stay Vigilant Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40 General Recommendations for Treating irAEs The Principles of irAEs Management Increasing intensity of treatment required Grade 2 Grade 1 Grade 3 Grade 4 Moderate Mild Severe Very severe Symptomatic and supportive therapy Stop treatment Oral steroids Intravenous steroids. ------------ • Referral to specialist • Strong immune suppressive treatment Increasing grade of irAE Intravenous steroids Steroids (PO/IV): 1-2 mg/kg/d prednisone or equivalent, slowly taper over 4-6 weeks For some AEs, treatment can be restarted after resolution (eg, rash); generally, ICI can be continued with endocrinopathies once managed Managed in outpatient/ community setting Generally requires hospital admission 01 Prevention 02 Anticipation 03 Detection 04 Treatment 05 Monitoring 01 02 03 04 05
- 9. Immune-Related Adverse Events of Cancer Immunotherapies1-17 Become Aware and Stay Vigilant Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40 Hold immunotherapy and reassess in 1-2 weeks Pulse oximetry rest and ambulation Consider chest imaging with CT (with contrast preferred) Repeat in 3-4 weeks Moderate (grade 2): 25%-50% lung involved Severe (grade 3-4) Grade 3: all lobes of lung or 50% of lung parenchyma; limited ADLs, oxygen requirement Grade 4: life-threatening Hold immunotherapy Infectious workup (nasal swab, sputum, blood) Consider bronchoscopy and BAL Chest imaging with CT contrast Repeat in 3-4 weeks Consider empiric antibiotics Refractory: methylprednisolone 1-2 mg/m2 /day; if no response in 3-4 days, treat as grade 3 Permanently discontinue immunotherapy and move to inpatient care Infectious workup (nasal swab, sputum, blood) Pulmonary and infectious disease consultation Bronchoscopy with BAL Empiric antibiotics Methylprednisolone 1-2 mg/m2 /day; when grade 1, taper over 6 weeks Refractory: infliximab, mycophenolate, or IVIG How Should Pulmonary irAEs Be Diagnosed and Managed? Pneumonitis: focal or diffuse inflammation of the lung parenchyma (typically identified on CT imaging) Diagnostic workup: CXR, CT, pulse oximetry; for grade ≥2, may include infectious workup Mild (grade 1): 25% lung involved Additional considerations • GI and pneumocystis prophylaxis may be offered to patients on prolonged steroid use (12 weeks) • Consider calcium and vitamin D supplementation with prolonged steroid use • Bronchoscopy and biopsy; if clinical picture is consistent with pneumonitis, no need for biopsy Supportive care: smoking cessation and vaccinations (influenza, pneumococcal)
- 10. Immune-Related Adverse Events of Cancer Immunotherapies1-17 Become Aware and Stay Vigilant Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40 Society for Immunotherapy of Cancer (SITC) Consensus Definitions for irAEs Recurrent irAEs • Occur in the same organ • Occur at least twice after IO discontinuation Steroid-unresponsive irAEs • No clinical improvement after a standard timeframe of guideline-based irAE-directed steroid therapy • Steroid-refractory irAEs derived no clinical benefit from steroids Steroid-resistant irAEs • Derived some clinical benefit without resolution of the event Steroid-dependent irAEs • Some improvement with guideline- based irAE-directed steroid therapy; however, a taper is not possible • irAEs requiring ongoing steroids for ≥12 weeks are “chronically steroid dependent” Delayed/late-onset irAEs • Occur 3 months after ICI discontinuation Chronic irAEs • Persist beyond 3 months of ICI discontinuation Two subtypes 1. Chronic + active: ongoing inflammation, requires ongoing immunosuppression 2. Chronic + inactive: absence of ongoing inflammation, not requiring ongoing immunosuppression Natural History of irAEs Multisystem irAEs • Occur concomitantly with another irAE or during treatment for the first irAE • irAEs occurring in the same or different organ system • If occurring in the same system, they affect different tissues Patterns of irAEs Response to irAE Treatment
- 11. Immune-Related Adverse Events of Cancer Immunotherapies1-17 Become Aware and Stay Vigilant Full abbreviations, accreditation, and disclosure information available at PeerView.com/JSU40 1. Ramos-Casals M et al. Nat Rev Dis Primers. 2020;6:38. 2. Martins F et al. Nat Rev Clin Oncol. 2019;16:563-580. 3. O’Leary CL et al. J Thorac Oncol. 2023 Oct 23 [Epub ahead of print]. 4. Helmink BA et al. Ann Surg Oncol. 2020;27:1533-1545. 5. Stiles BM et al. J Thorac Cardiovasc Surg. 2020;160:1376-1382. 6. Champiat S et al. Ann Oncol. 2016;27:559-574. 7. Brahmer JR et al. J Clin Oncol. 2018;36:1714-1786. 8. https://www.esmo.org/content/download/124130/2352601/1/ESMO-Patient-Guide-on-Immunotherapy-Side-Effects.pdf. 9. https://www.nccn.org/ professionals/physician_gls/pdf/immunotherapy.pdf. 10. Puzanov I et al. J Immunother Cancer. 2017;5:95. 11. Brahmer JR et al. J Clin Oncol. 2018;36:1714-1786. 12. Provided courtesy of Marianne Davies, DNP, ACNP, AOCNP, FAAN, 2021; adapted from AIM with Immunotherapy, NCCN, and CTCAE. 13. Naidoo J et al. J Immunother Cancer. 2023;11:e006398. 14. https://ascopubs.org/doi/full/10.1200/JCO.21.01440. 15. https://www.esmo.org/content/download/124130/2352601/1/ESMO-Patient-Guide-on-Immunotherapy-Side-Effects.pdf. 16. https://www.nccn.org/ professionals/physician_gls/pdf/immunotherapy.pdf. 17. https://www.sitcancer.org/research/cancer-immunotherapy-guidelines/irae/immune-checkpoint-inhibitor-related-adverse-events. Additional Guideline Recommendations for Treating irAEs
