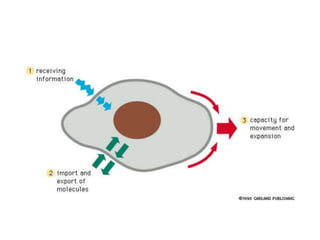
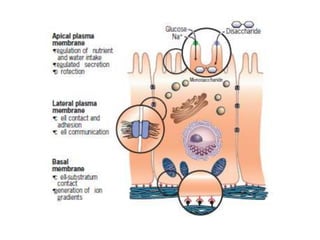
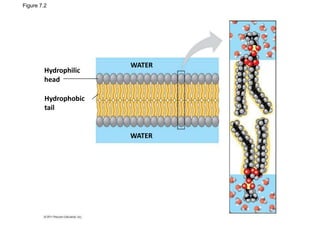
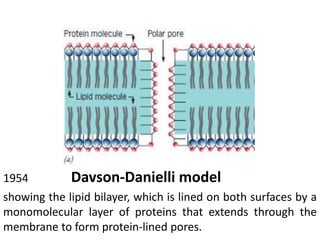
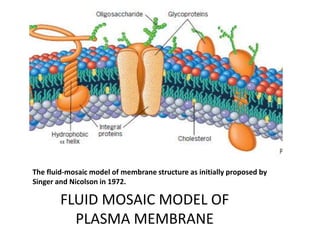
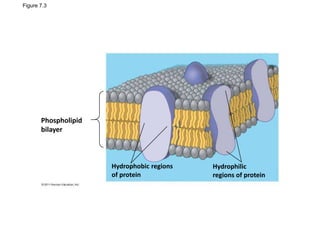
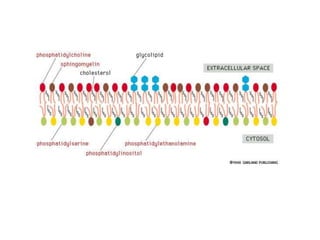
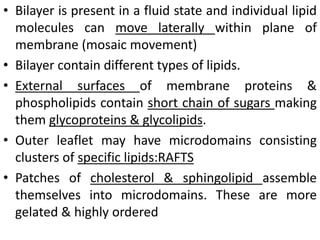
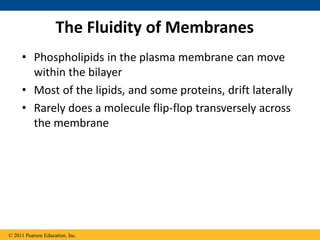
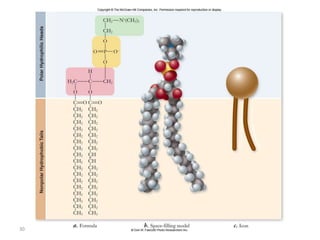
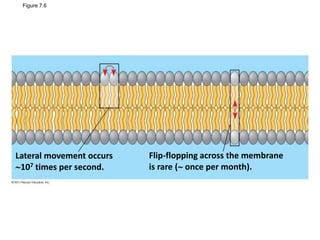
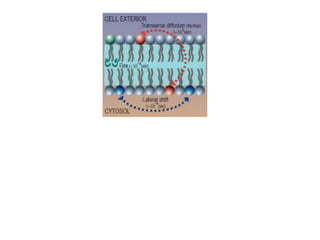
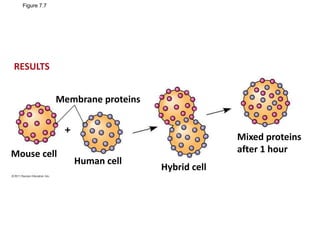
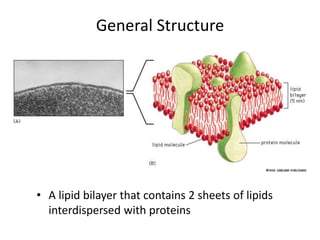
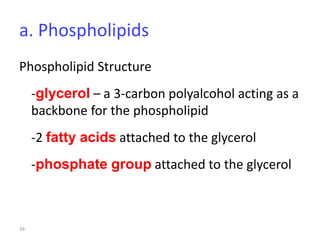
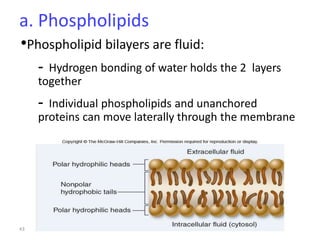
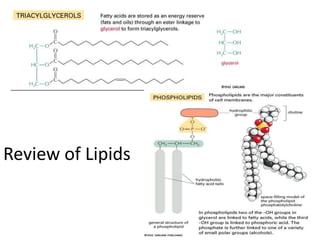
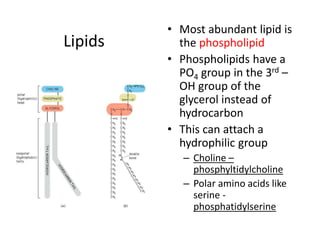
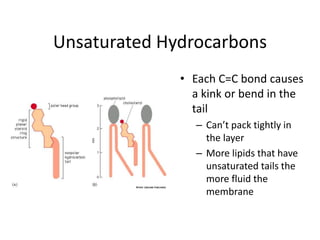
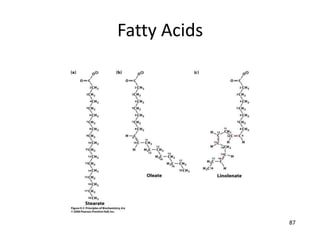
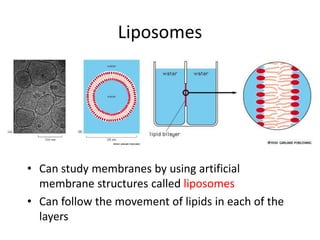
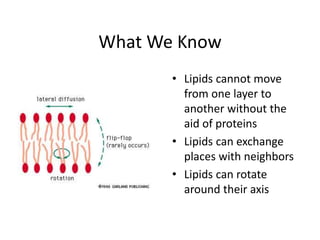
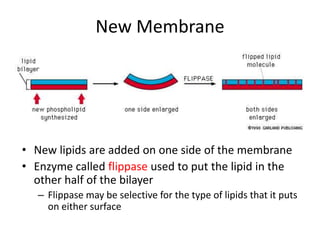
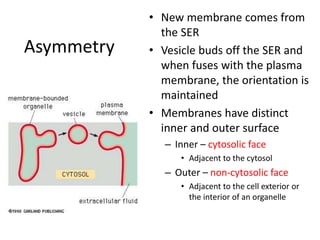
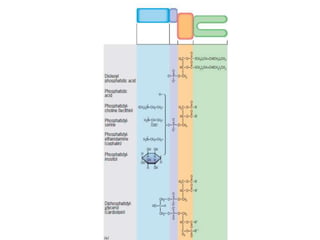
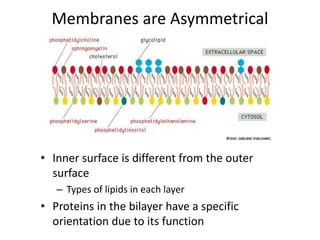
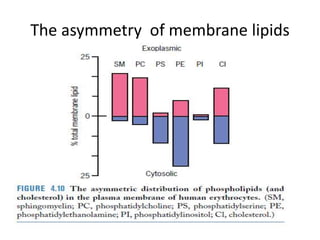
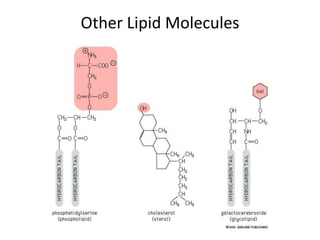
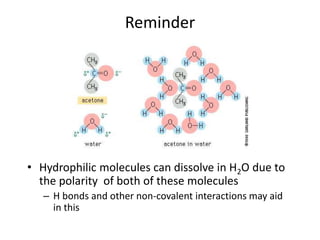
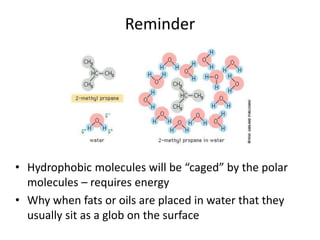
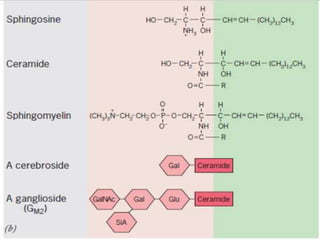
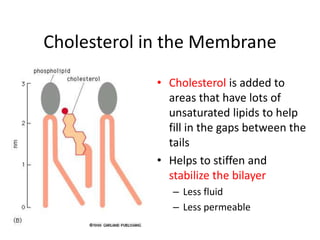
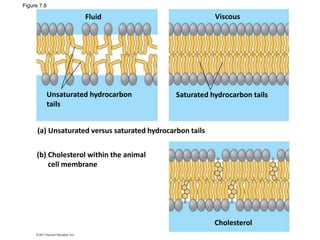
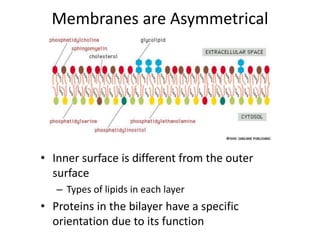
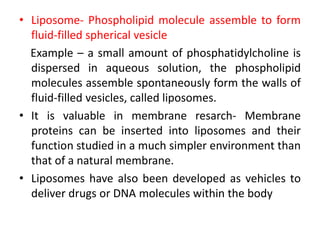
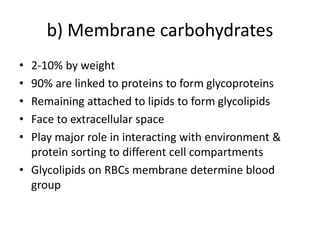
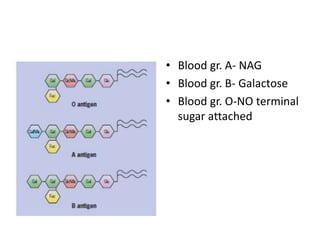
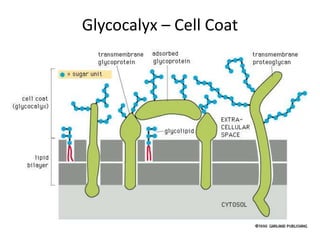
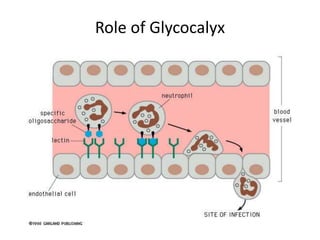
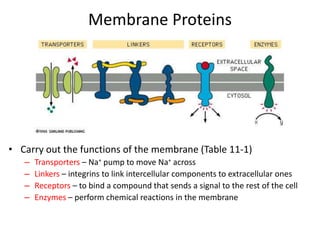
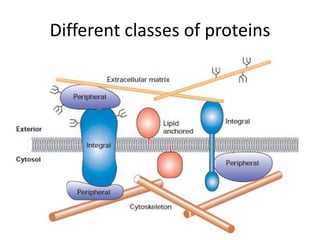
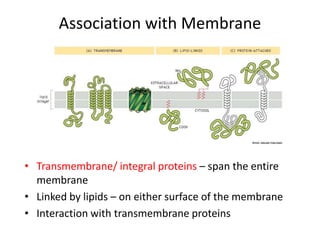
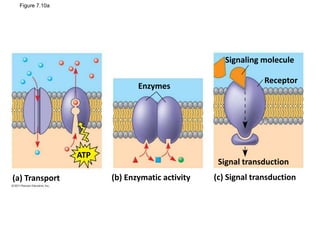
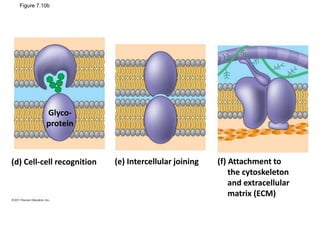
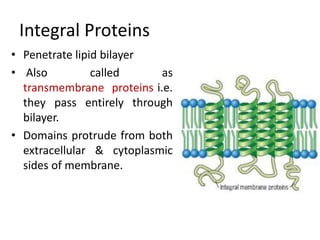
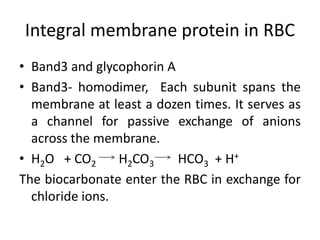
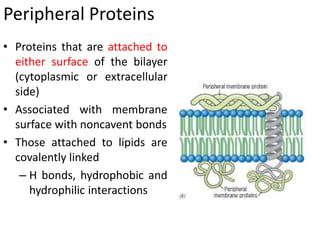
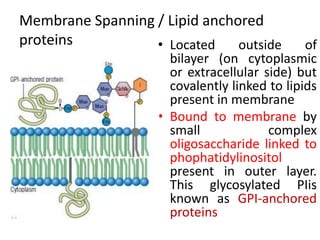
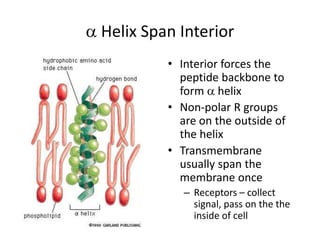
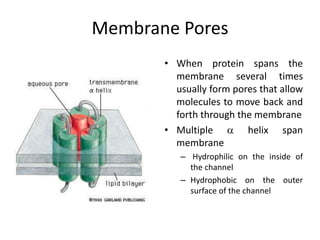
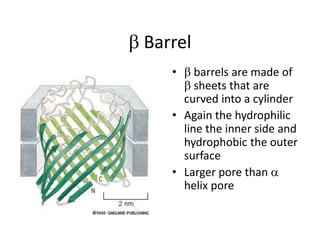
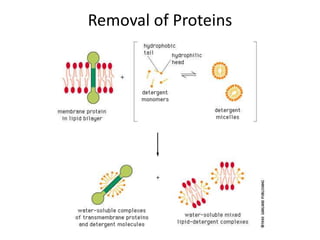
The document discusses membrane structures, specifically plasma membranes. It begins by explaining that plasma membranes hold the cell together and act as a barrier, being composed of a phospholipid bilayer with embedded and peripheral proteins. It then provides details on the fluid mosaic model of membrane structure, which proposes that membranes are a fluid bilayer of lipids with globular proteins dispersed within. The functions of plasma membranes are then outlined, including compartmentalization, selectively permitting transport, responding to signals, and mediating cell-cell interactions through receptors.