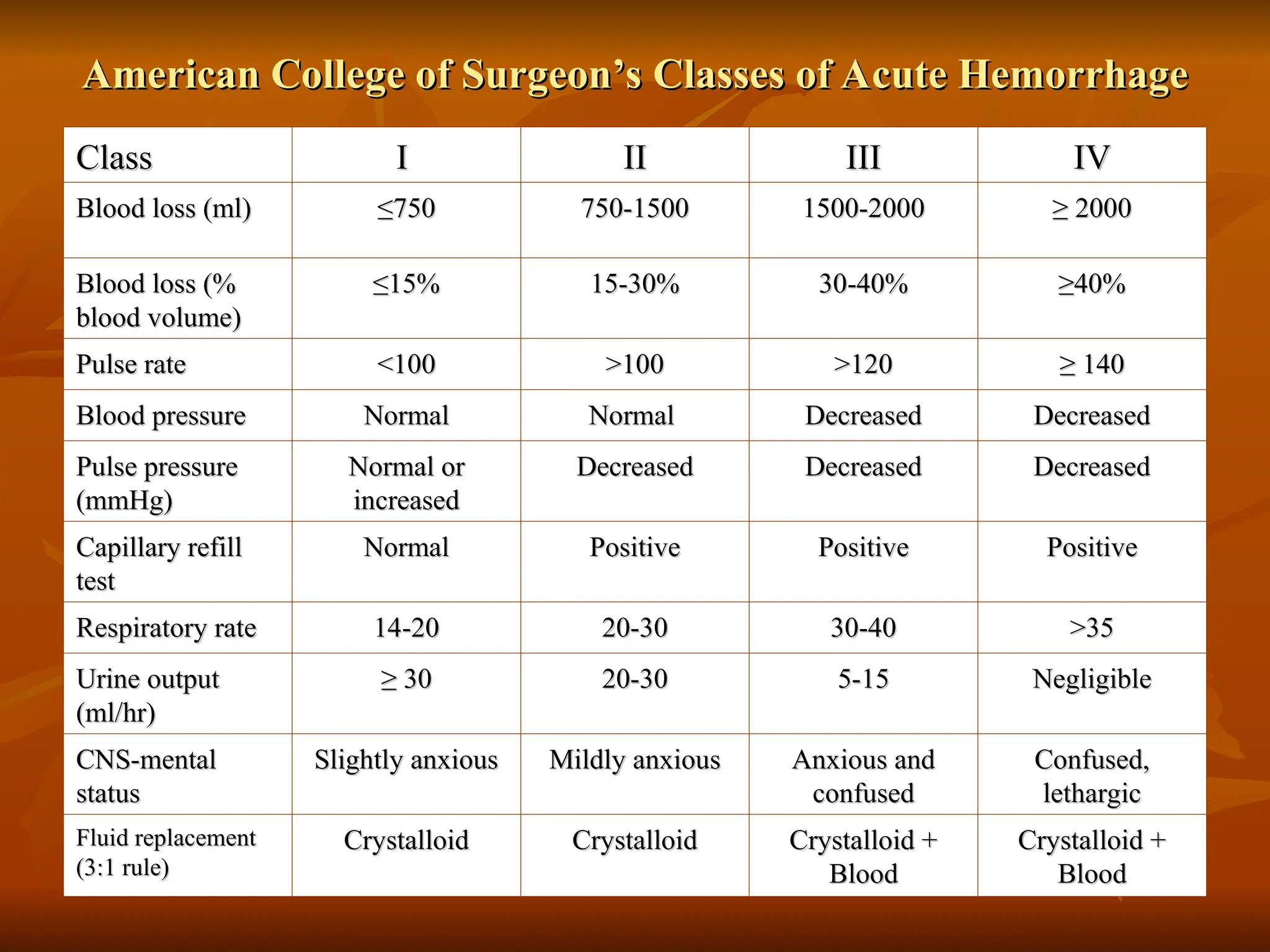
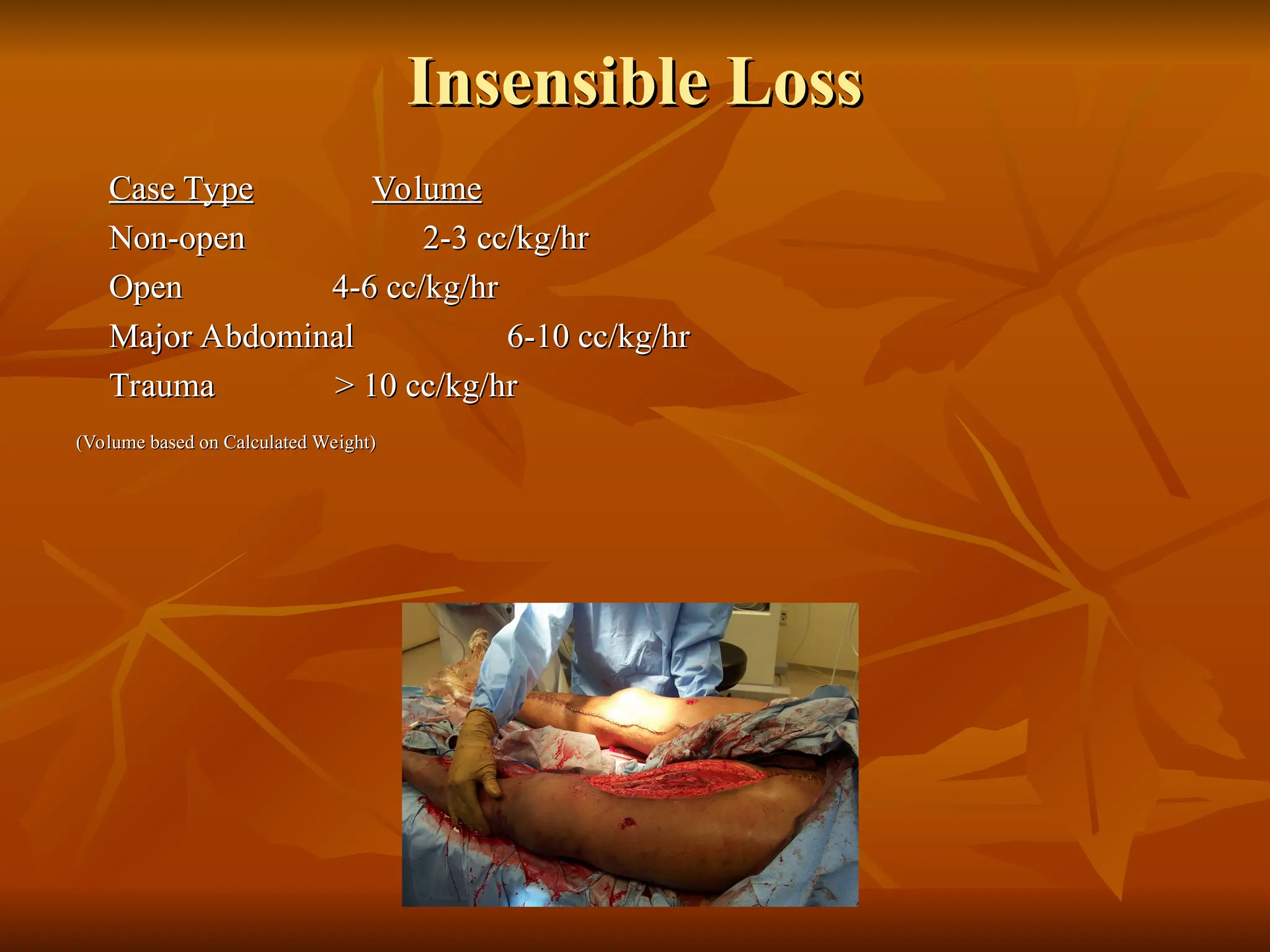
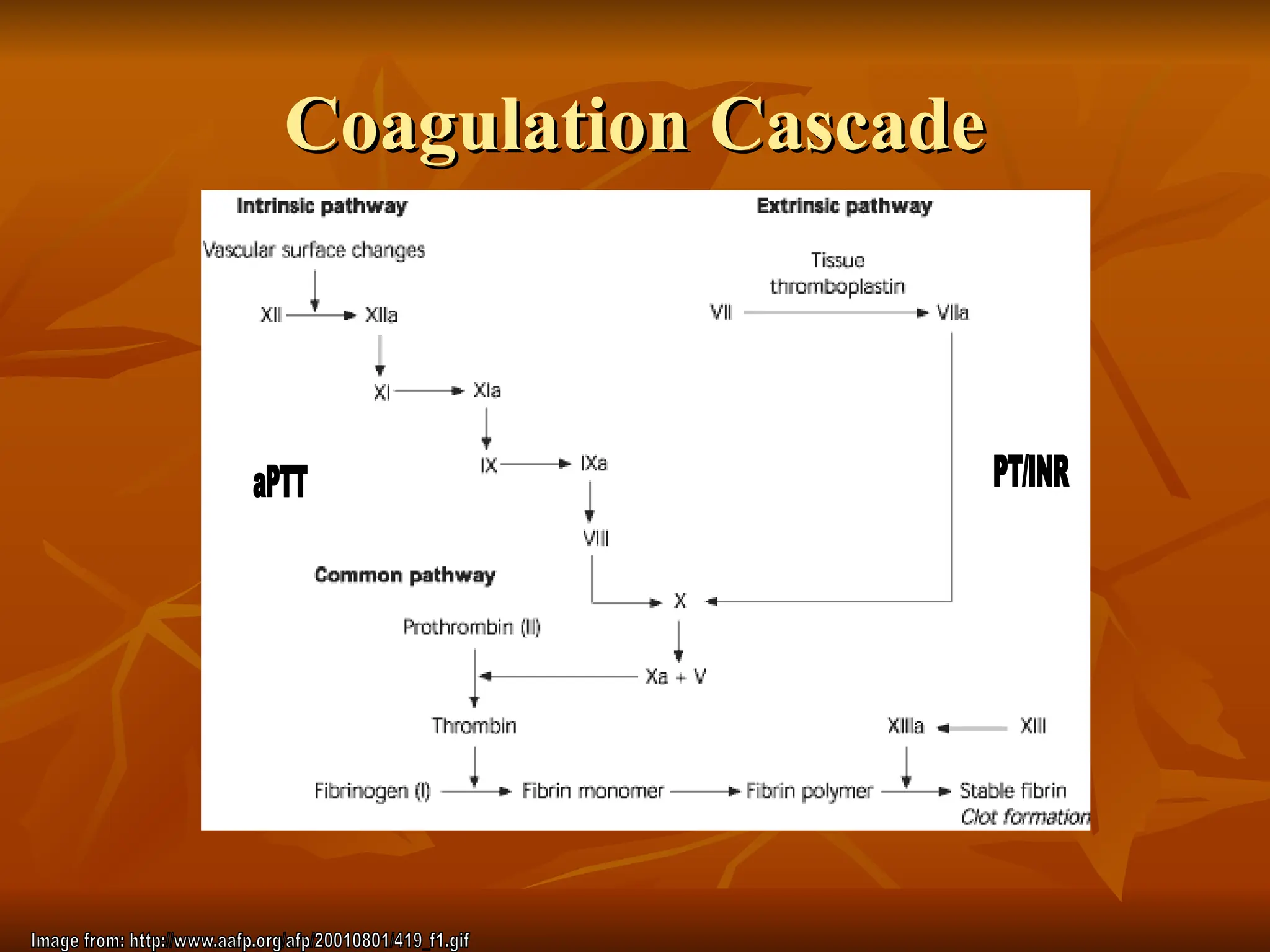
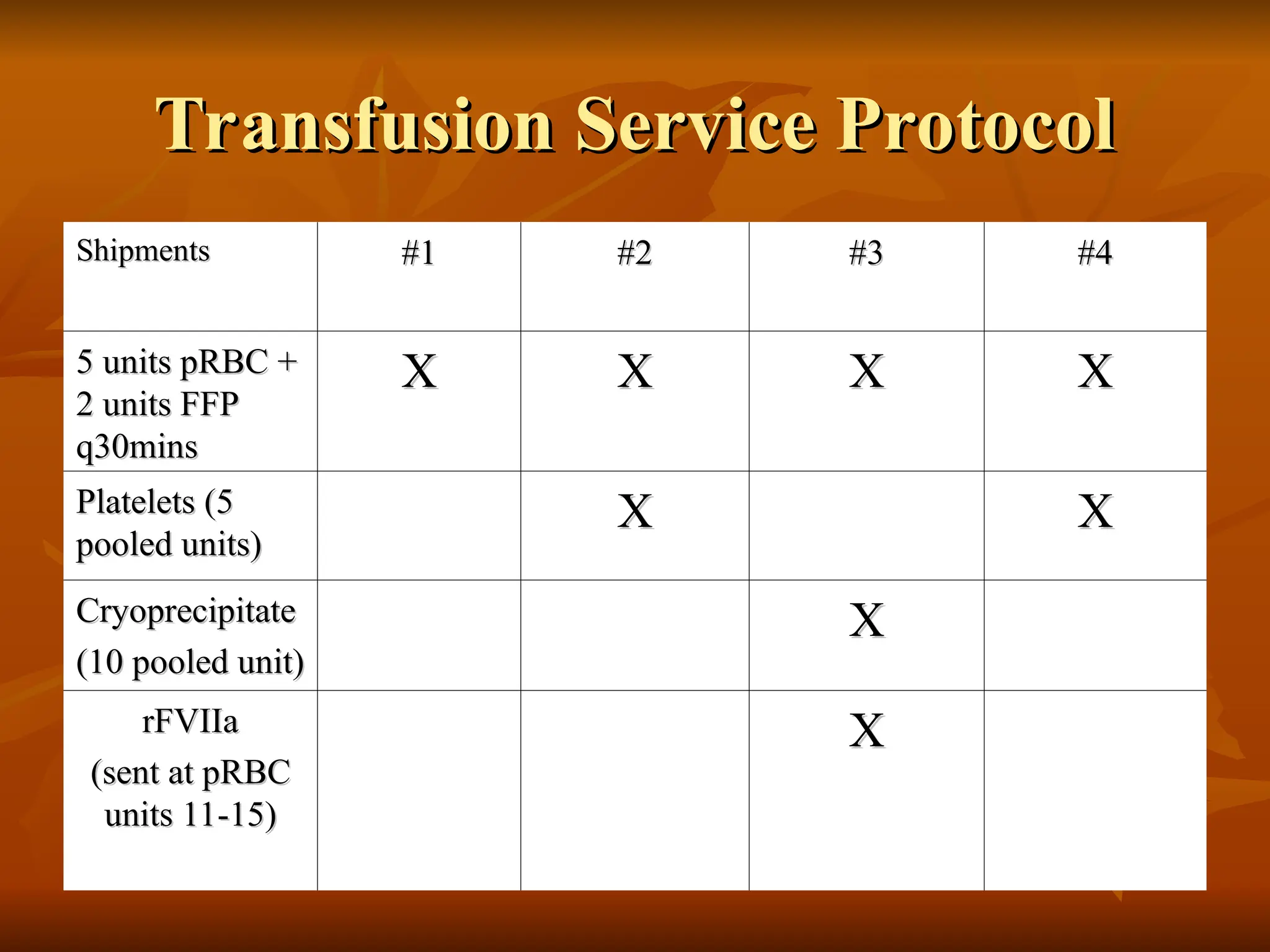
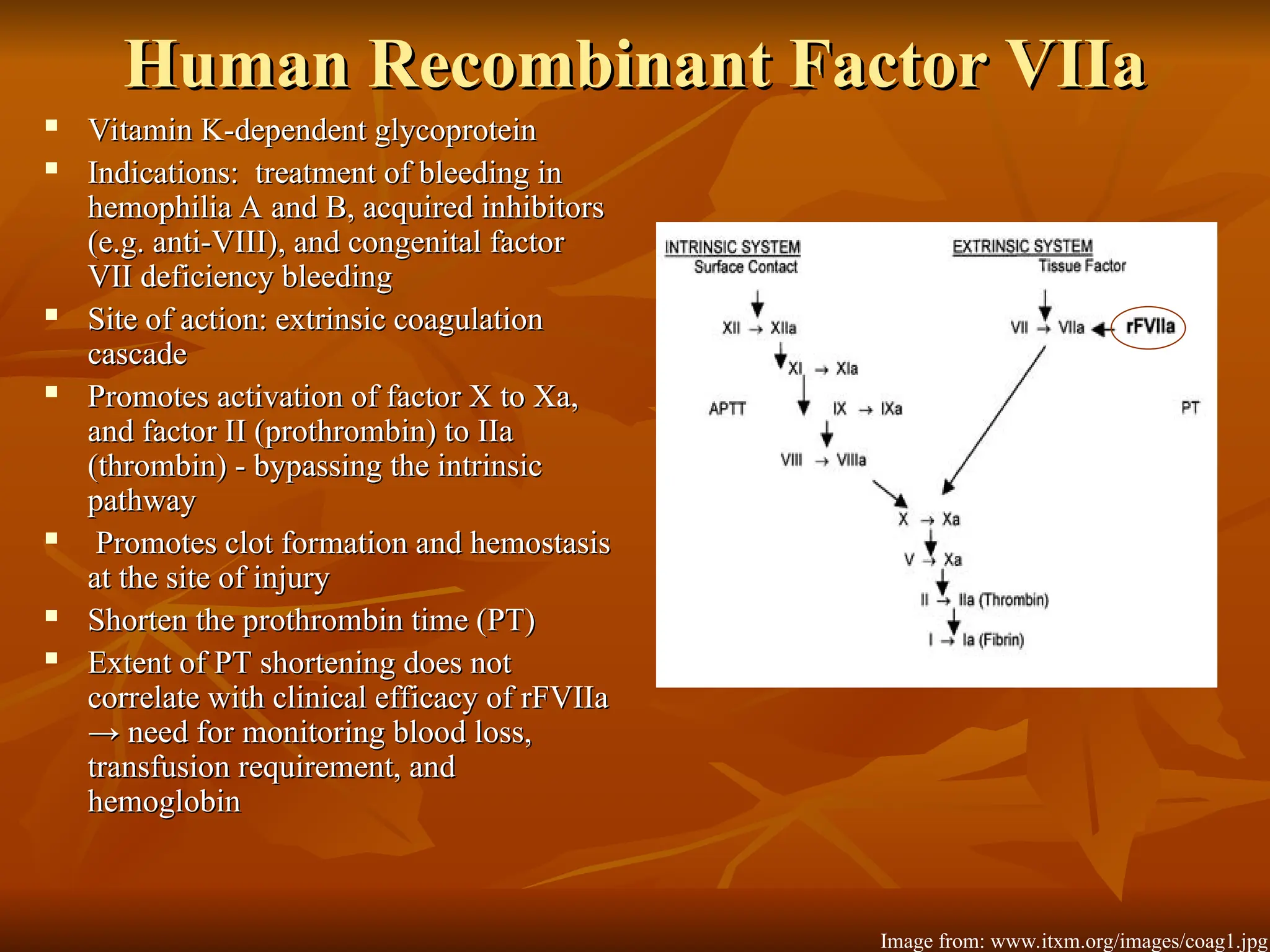
The document discusses massive transfusion and coagulopathy, highlighting guidelines for blood product transfusions established by the ASA in 1994. It covers the definition and classes of acute hemorrhage, fluid replacement strategies, and different blood components used in transfusions. Key considerations include the benefits and risks associated with transfusions, requirements for blood product compatibility, and the impact of coagulopathy in massive transfusions.