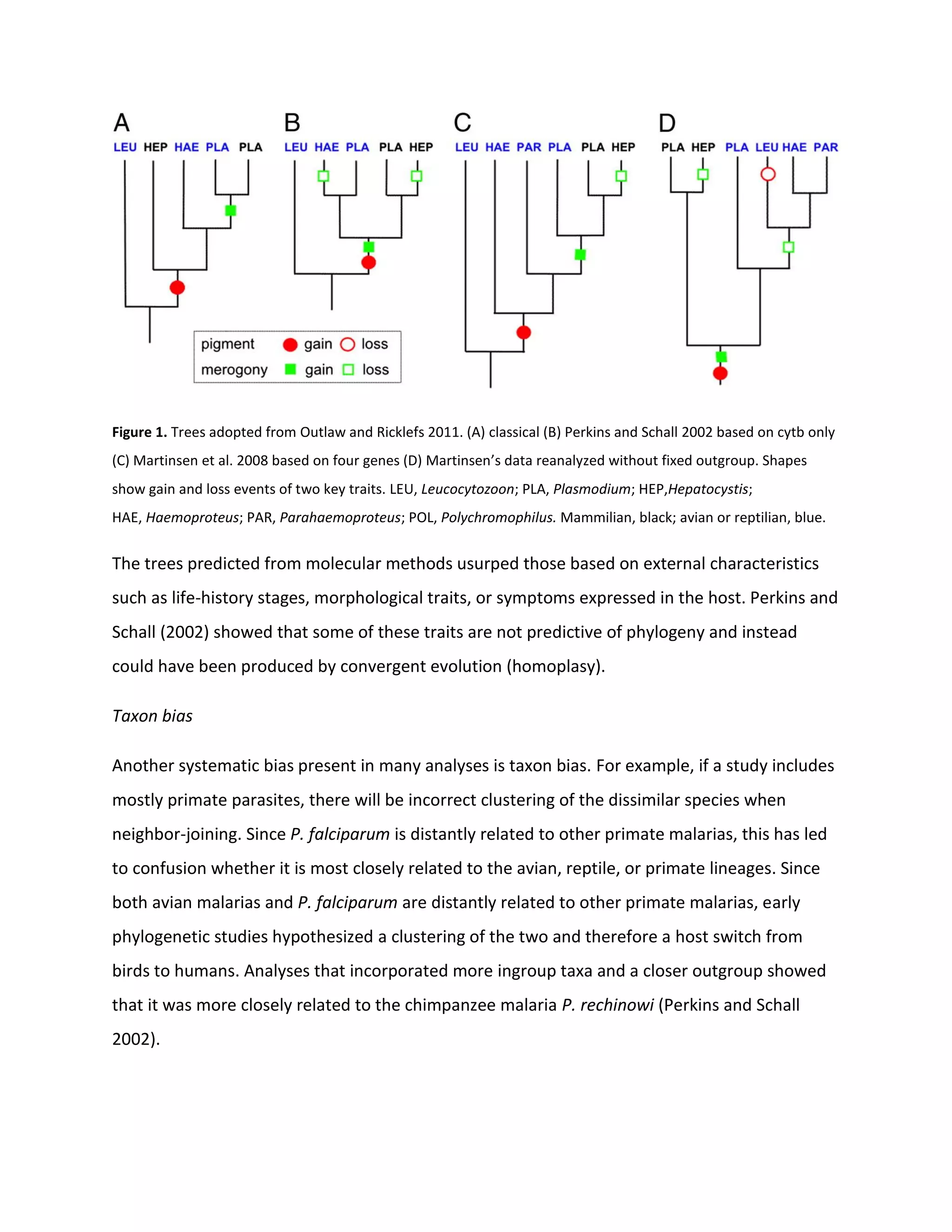
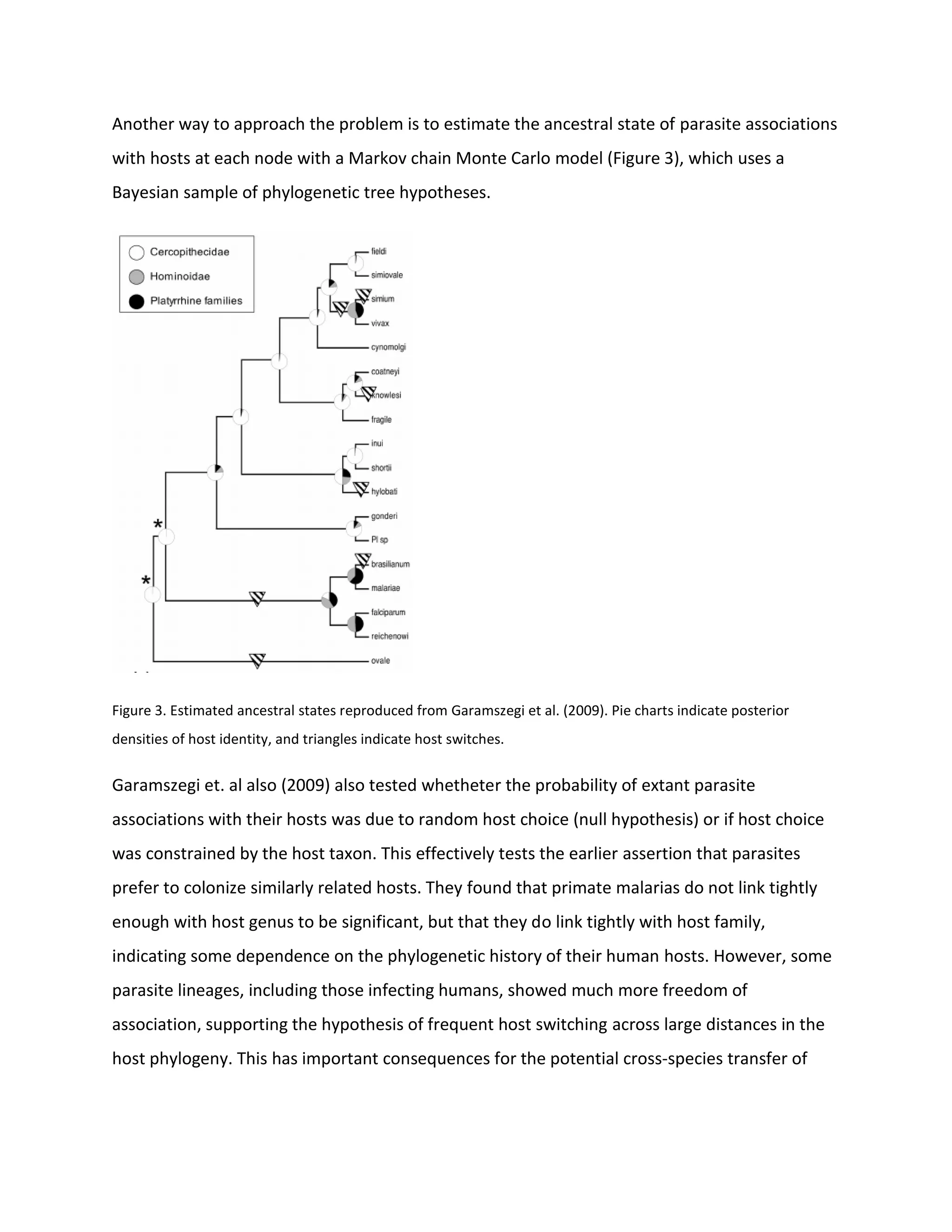
1. The evolutionary relationships between malaria parasite species have been controversial due to past studies relying on visible traits rather than molecular data and issues like taxon bias.
2. Different genes are suitable for phylogenetic analysis, with some like rRNA being problematic due to paralogs. Studies using multiple genes from different genomic compartments provide better resolution.
3. The origin of P. falciparum, which causes the most virulent human malaria, has been debated, with evidence it may have recently switched hosts from gorillas rather than co-diverging with humans. Further sampling of ape malarias is needed to resolve this.








![References
Bensch, Staffan, Olof Hellgren, Asta Križanauskienė, Vaidas Palinauskas, Gediminas Valkiūnas,
Diana Outlaw, and Robert E. Ricklefs. 2013. How Can We Determine the Molecular Clock of
Malaria Parasites? Trends in Parasitology 29 (8): 363–69. doi:10.1016/j.pt.2013.03.011.
Garamszegi, László Z. 2009. Patterns of Co-Speciation and Host Switching in Primate Malaria
Parasites. Malaria Journal 8 (1): 110. doi:10.1186/1475-2875-8-110.
Huelsenbeck, John P., Jonathan P. Bollback, and Amy M. Levine. 2002. Inferring the Root of a
Phylogenetic Tree. Systematic Biology 51 (1): 32–43. doi:10.1080/106351502753475862.
Hughes, Austin L., and Federica Verra. 2010. Malaria Parasite Sequences from Chimpanzee
Support the Co-Speciation Hypothesis for the Origin of Virulent Human Malaria (Plasmodium
Falciparum). Molecular Phylogenetics and Evolution 57 (1): 135–43.
doi:10.1016/j.ympev.2010.06.004.
Hughes, M. K., and A. L. Hughes. 1995. Natural Selection on Plasmodium Surface Proteins.
Molecular and Biochemical Parasitology 71 (1): 99–113.
Liu, Weimin, Yingying Li, Gerald H. Learn, Rebecca S. Rudicell, Joel D. Robertson, Brandon F.
Keele, Jean-Bosco N. Ndjango, et al. 2010. Origin of the Human Malaria Parasite Plasmodium
Falciparum in Gorillas. Nature 467 (7314): 420–25. doi:10.1038/nature09442.
Martinsen, Ellen S., Susan L. Perkins, and Jos J. Schall. 2008. A Three-Genome Phylogeny of
Malaria Parasites (Plasmodium and Closely Related Genera): Evolution of Life-History Traits and
Host Switches. Molecular Phylogenetics and Evolution 47 (1): 261–73.
doi:10.1016/j.ympev.2007.11.012.
Outlaw, Diana C., and Robert E. Ricklefs. 2011. Rerooting the Evolutionary Tree of Malaria
Parasites. Proceedings of the National Academy of Sciences 108 (32): 13183–87.
doi:10.1073/pnas.1109153108.
Pacheco, M Andreína, Fabia U Battistuzzi, Randall E Junge, Omar E Cornejo, Cathy V Williams,
Irene Landau, Lydia Rabetafika, Georges Snounou, Lisa Jones-Engel, and Ananias A Escalante.
2011. Timing the Origin of Human Malarias: The Lemur Puzzle. BMC Evolutionary Biology 11
(October): 299. doi:10.1186/1471-2148-11-299.
Perkins, Susan L., and JosJ. Schall. 2002. A molecular phylogeny of malarial parasites recovered
from cytochrome b gene sequences. Journal of Parasitology 88 (5): 972–78. doi:10.1645/0022-
3395(2002)088[0972:AMPOMP]2.0.CO;2.
Pevzner, Pavel, and Ron Shamir. 2011. Bioinformatics for Biologists. Cambridge University Press.](https://image.slidesharecdn.com/4dfcca67-fa3b-4c8a-a0d2-8e0983c00b7a-150709224921-lva1-app6892/75/malaria_paper-11-2048.jpg)
